Cybin (CYBN) Begins Phase II Study on Anxiety Drug, Stock Up
Cybin Inc.’s CYBN shares were up 7.2% on Mar 15 after the company initiated a phase II proof-of-concept study evaluating its proprietary deuterated dimethyltryptamine molecule, CYB004, for the treatment of patients with generalized anxiety disorder (GAD).
Per the company, the double-blind phase II CYB004-002 study will investigate the safety and efficacy of CYB004 in the given patient population. Patients will be divided into two groups. The first group of patients will receive two IM doses of CYB004, while the second group will take two low-dose control administrations of CYB004.
The primary endpoint of the study is to see the change in the Hamilton Anxiety Rating Scale score from baseline at six weeks of treatment after the administration of the second dose.
Meanwhile, the Montgomery-Asberg Depression Rating scale depression assessment, safety assessments, psychedelic experience assessment and the quality of life assessment remain the other endpoints of the above study.
Top-line data from the CYB004-002 study is expected to be announced in the fourth quarter of 2024. Data from the study is likely to establish the proof of concept for the efficacy of CYB004 in the treatment of GAD.
The FDA cleared the investigational new drug application for CYB004 in January 2024.
Shares of Cybin have rallied 24.4% in the past year against the industry’s decline of 7.7%.
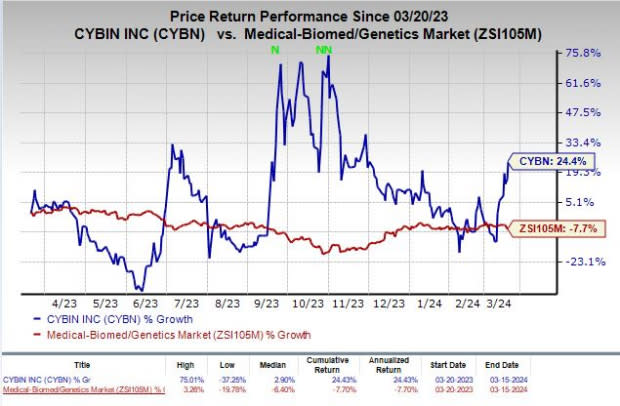
Image Source: Zacks Investment Research
Another company that is developing a treatment for GAD is Mind Medicine (MindMed) (MNMD).
MNMD plans to initiate a phase III study evaluating its lead candidate, MM120, for treating GAD.
Earlier this month, the FDA granted breakthrough therapy designation to MM120 for the given indication.
This apart, Cybin is developing another candidate, CYB003, in a phase II study for the treatment of major depressive disorder (MDD).
The FDA recently granted a breakthrough therapy designation to CYB003 for treating MDD. The company held a positive end-of-phase II meeting with the regulatory body to discuss the path ahead for CYB003 and announced positive four-month durability data, which support a phase III study on CYB003, which is likely to begin in the middle of 2024.
Zacks Rank & Stocks to Consider
Cybin currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the healthcare sector are ADMA Biologics, Inc. ADMA, Vanda Pharmaceuticals Inc. VNDA and ANI Pharmaceuticals, Inc. ANIP, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ADMA Biologics’ 2024 earnings per share have improved from 22 cents to 30 cents. In the past year, shares of ADMA have rallied 94.5%.
ADMA Biologics’ earnings beat estimates in three of the trailing four quarters and met the same once. ADMA delivered an average earnings surprise of 85.00%.
In the past 60 days, the Zacks Consensus Estimate for Vanda Pharmaceuticals’ 2024 bottom line has improved from a loss of 46 cents per share to earnings of 1 cent. In the past year, shares of VNDA have plunged 42.3%.
Vanda Pharmaceuticals’ earnings beat estimates in each of the trailing three quarters. VNDA delivered an average earnings surprise of 92.88%.
In the past 60 days, estimates for ANI Pharmaceuticals’ 2024 earnings per share have improved from $4.06 to $4.40. In the past year, shares of ANIP have surged 67.7%.
Earnings of ANI Pharmaceuticals beat estimates in each of the trailing four quarters. ANIP delivered a four-quarter average earnings surprise of 109.60%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vanda Pharmaceuticals Inc. (VNDA) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Cybin Inc. (CYBN) : Free Stock Analysis Report
