Cytokinetics (CYTK) Up 83% as Cardiomyopathy Drug Meets Goals
Cytokinetics, Inc. CYTK, a clinical-stage company, announced positive top-line results from its pivotal late-stage study of its investigational candidate, aficamten, in patients with symptomatic obstructive hypertrophic cardiomyopathy (HCM).
Cytokinetics’ aficamten is a small molecule cardiac myosin inhibitor with next-in-class potential in the treatment of debilitating diseases that hamper muscle functioning. The candidate currently enjoys the FDA’s Breakthrough Therapy designation in the United States for the treatment of symptomatic obstructive HCM.
In the pivotal phase III SEQUOIA-HCM study, treatment with aficamten resulted in a statistically significant improvement in exercise capacity compared with placebo, demonstrating an increase in peak oxygen uptake, as measured by cardiopulmonary exercise testing at week 24. This was also the primary efficacy endpoint of the study.
It was also observed that the positive treatment effect with aficamten was consistent across all subgroups of patients, including those receiving or not receiving background beta-blocker therapy.
CYTK’s stock skyrocketed 82.5% in the last trading session as the investors cheered the encouraging results from the pivotal SEQUOIA-HCM study of aficamten in symptomatic obstructive HCM patients. Year to date, shares of the company have rallied 82.1% against the industry’s 15.1% decline.
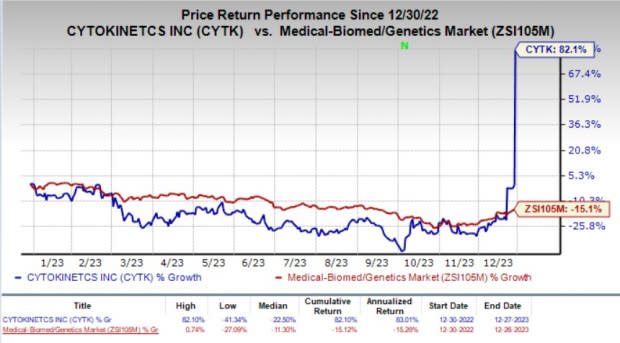
Image Source: Zacks Investment Research
Cytokinetics further reported that the pivotal SEQUOIA-HCM study also met all ten prespecified secondary endpoints with statistical significance, demonstrating clinically meaningful improvements in symptomatic obstructive HCM patients treated with aficamten.
The drug was overall well tolerated with an adverse event profile comparable to placebo. The company will share detailed results from the pivotal SEQUOIA-HCM study at an upcoming medical conference in 2024.
The company is also simultaneously evaluating aficamten for two other muscle disease indications. A phase III MAPLE-HCM study is currently evaluating aficamten as monotherapy compared with metoprolol as monotherapy in patients with obstructive HCM. Another ongoing phase III ACACIA-HCM study is evaluating aficamten in patients with symptomatic non-obstructive HCM.
HCM is a kind of heart disease that causes the abnormal thickening of the heart muscle, resulting in constricted blood flow in the heart, which, in turn, limits the heart’s pumping function. This ultimately results in reduced exercise capacity and other symptoms, which include chest pain, dizziness, shortness of breath, or fainting during physical activity.
Cytokinetics has another candidate in its late-stage pipeline, omecamtiv mecarbil, a cardiac myosin activator, which is being developed to treat heart failure with reduced ejection fraction.
Beyond these late-stage candidates, CYTK’s pipeline comprises two other investigational drugs, CK-136 and CK-586, which are currently being developed in two separate early-stage studies for the treatment of different heart failure indications.
Currently, Bristol Myers BMY markets the first and only FDA-approved drug for the treatment of adults with symptomatic obstructive HCM to improve functional capacity and symptoms. BMY’s Camzyos (mavacamten, 2.5 mg, 5 mg, 10 mg, 15 mg capsules) received approval last year for this indication.
It is an allosteric and reversible inhibitor selective for cardiac myosin that targets the underlying pathophysiology of obstructive HCM. Camzyos is also currently marketed by Bristol Myers in the EU as the first and only medication of its kind for the same indication as in the United States.
However, it is important to note that the label for Bristol Myers’ Camzyos includes a boxed warning for the risk of heart failure.
Cytokinetics, Incorporated Price and Consensus
Cytokinetics, Incorporated price-consensus-chart | Cytokinetics, Incorporated Quote
Zacks Rank and Stocks to Consider
Cytokinetics currently has a Zacks Rank #4 (Sell).
A few better-ranked drug/biotech stocks worth mentioning are Puma Biotechnology, Inc. PBYI and ADMA Biologics ADMA. While PBYI sports a Zacks Rank #1 (Strong Buy), ADMA carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has decreased from 73 cents to 72 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 62 cents to 64 cents. In the year so far, shares of PBYI have gained 2.6%.
PBYI’s earnings beat estimates in three of the last four quarters while missing on one occasion, delivering a four-quarter average earnings surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 3 cents to 2 cents. The consensus estimate for ADMA Biologics’ 2024 EPS is pegged at 18 cents. In the year so far, shares of ADMA have gained 15.7%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 63.57%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Cytokinetics, Incorporated (CYTK) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
