Deciphera (DCPH) Issues Strategic & Corporate Outlook for 2023
Deciphera Pharmaceuticals, Inc. DCPH announced an outlook and planned corporate milestones for 2023. The company outlined certain commercial, clinical and preclinical activities for the year.
Along with the same press release, DCPH announced preliminary revenues for the fourth quarter and 2022. The company expects preliminary revenues of $36 million and $134 million for the fourth quarter and 2022, respectively.
Shares of Deciphera have rallied 64.7% in the past year against the industry’s decline of 17.4%.
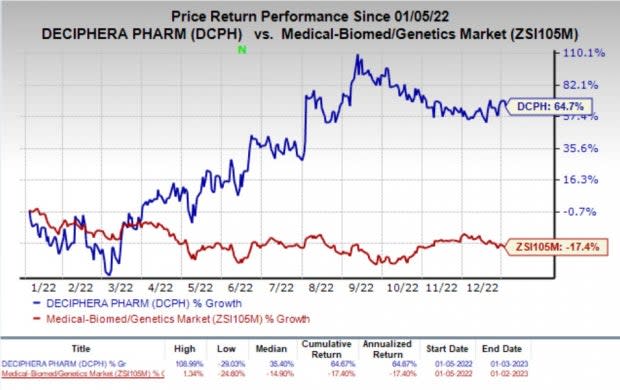
Image Source: Zacks Investment Research
Per the outlook for 2023, the company plans to initiate the pivotal phase III INSIGHT study evaluating its sole marketed drug, Qinlock (ripretinib), compared with sunitinib for treating patients with gastrointestinal stromal tumor (GIST) who were previously treated with Novartis’ NVS Gleevec (imatinib) in the second half of 2023.
NVS lost patent protection for Gleevec, which faces increasing generic competition in major markets.
In a separate press release, DCPH announced data from the phase III INTRIGUE study evaluating Qinlock using circulating tumor DNA (ctDNA) from a subgroup of patients with GIST previously treated with Gleevec who harbor mutations in KIT exon 11 and 17/18 only. The study compared Qinlock to sunitinib for treating second-line GIST patients with mutations in KIT exon 11 and 17/18 only.
Data from the same showed that patients treated with Qinlock had median progression-free survival of 14.2 months versus 1.5 months for patients receiving sunitinib. The objective response rate was 44.4% in the Qinlock arm versus nil in the sunitinib arm.
Qinlock is approved in the United States for the treatment of adult patients with advanced GIST who have received prior treatment with three or more kinase inhibitors, including Novartis’ Gleevec.
Apart from this, Deciphera is evaluating its pipeline candidate vimseltinib in the phase III MOTION study to treat patients with tenosynovial giant-cell tumors, who are not amenable to surgery. Enrollment in the study is expected to be completed in the first half of 2023, with top-line data from the same expected in the fourth quarter of 2023.
DCPH plans to evaluate DCC-3116 in combination with Pfizer’s PFE Braftovi (encorafenib) and Eli Lilly’s Erbitux (cetuximab) for treating patients with colorectal cancer in the second half of 2023.
Deciphera entered a clinical trial collaboration and supply agreement with Pfizer for Braftovi.
Per the above agreement, Deciphera will sponsor the study, whereas PFE will supply Braftovi free of cost.
DCPH plans to file an investigational new drug application for DCC-3084, a potential best-in-class pan-RAF inhibitor, in the second half of 2023 with the FDA.
Zacks Rank & Stock to Consider
Deciphera currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the biotech sector is Syndax Pharmaceuticals, Inc. SNDX, which sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Syndax Pharmaceuticals have narrowed 14.5% for 2023 in the past 60 days.
Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Deciphera Pharmaceuticals, Inc. (DCPH) : Free Stock Analysis Report
