Editas (EDIT) Gets FDA's RMAT Tag for Gene Therapy to Treat SCD
Editas Medicine, Inc. EDIT announced that its investigational gene-editing candidate, EDIT-301, received Regenerative Medicine Advanced Therapy (“RMAT”) designation from the FDA. Shares gained 3% on the news.
The regulatory body granted the RMAT tag to EDIT-301 for the treatment of severe sickle cell disease (SCD), an inherited blood disorder that leads to anemia and early death.
The RMAT designation is generally granted to therapies that are intended to treat, or cure a serious or life-threatening disease, and have the potential to address unmet medical needs.
The RMAT designation also opens up early interactions between the FDA and sponsors to facilitate accelerated approval and potential priority review of a product’s biologics license application.
We remind investors that Editas is also developing EDIT-301 for the treatment of transfusion-dependent beta thalassemia (TDT).
The FDA has already granted the Orphan Drug Designation to EDIT-301 for the treatment of SCD and for the treatment of beta thalassemia. The regulatory body has also bestowed Rare Pediatric Disease designation to EDIT-301 for the treatment of beta thalassemia and SCD.
The FDA’s orphan drug designation for both these indications will grant EDIT-301 market exclusivity in the United States upon potential approval.
Shares of Editas have plunged 18.2% so far this year compared with the industry’s decline of 18.9%.
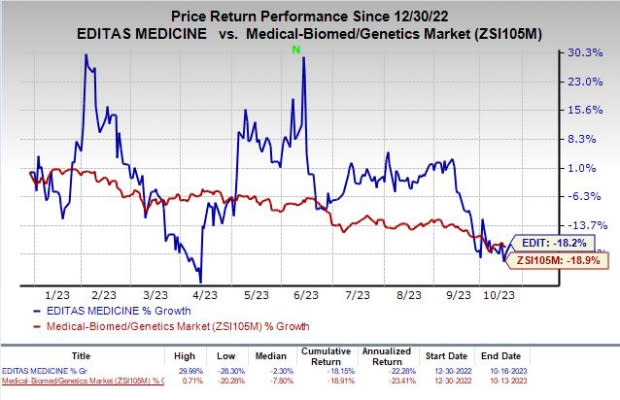
Image Source: Zacks Investment Research
EDIT is currently evaluating the safety and efficacy of EDIT-301 in the RUBY study for treating SCD. The company remains on track to provide an additional clinical update from the RUBY study by 2023-end.
Meanwhile, parallel dosing in the phase I/II EDITHAL study is currently underway. Editas plans to provide an additional clinical update from the EDITHAL study by the end of the ongoing year.
In June 2023, Editas announced positive preliminary safety and efficacy data from the first four patients with SCD treated with EDIT-301 in the RUBY study and the first TDT patient treated in the EDITHAL study.
Per the company, treatment with EDIT-301 was well-tolerated by all four patients in the RUBY study, as well as, the first patient in the EdiTHAL study.
Editas has no approved products in its portfolio at the moment. Therefore, pipeline development remains the key focus of the company.
Zacks Rank & Stocks to Consider
Editas currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Neurocrine Biosciences, Inc. NBIX, Sarepta Therapeutics, Inc. SRPT and MEI Pharma, Inc. MEIP, sporting a Zacks Rank #1 (Strong Buy) each. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates per share for Neurocrine Biosciences 2023 have risen from $2.17 to $2.19. During the same period, estimates per share estimates for 2024 have risen from $4.77 to $4.86. Year to date, shares of NBIX have lost 4.9%.
Earnings of Neurocrine Biosciences beat estimates in one of the last four quarters and missed the same on the other three occasions. NBIX delivered a four-quarter negative average earnings surprise of 105.45%.
In the past 60 days, estimates for Sarepta’s 2023 loss per share have improved from $9.73 to $9.19. During the same period, loss per share estimates for 2024 have narrowed from $1.66 to $1.00. Year to date, shares of SRPT have declined 9.3%.
Earnings of Sarepta Therapeutics beat estimates in three of the trailing four quarters and missed the mark on the other occasion. On average, SRPT delivered a negative earnings surprise of 5.15% in the last four quarters.
In the past 60 days, estimates for MEI Pharma’s 2023 loss per share have improved from $6.54 to $4.89. During the same period, the loss per share estimates for 2024 have narrowed from $5.14 to $4.02. Year to date, shares of MEIP have rallied 40.4%.
Earnings of MEI Pharma beat estimates in three of the trailing four quarters and met the same on the other occasion. On average, MEIP came up with an average four-quarter earnings surprise of 53.58%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Neurocrine Biosciences, Inc. (NBIX) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
MEI Pharma, Inc. (MEIP) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
