Editas (EDIT) Licenses Cas9 Tool to Vertex, Stock Up 6%
Editas Medicine, Inc. EDIT announced entering into a licensing agreement with Vertex Pharmaceuticals VRTX for the development of the latter’s new sickle cell disease (SCD) gene therapy, Casgevy.
Per the terms of the agreement, Vertex will receive a non-exclusive license to utilize Editas’ Cas9 gene editing technology for ex vivo gene editing medicines targeting the BCL11A gene in the fields of sickle cell disease and beta thalassemia, including Casgevy (exagamglogene autotemcel [exa-cel]).
The Cas9 gene editing tool will provide Vertex access to a broad range of genetic mutations, which makes it possible to develop innovative gene-editing medicines with a novel mechanism of action.
Financial considerations for the transaction have not been stated by the companies. However, the deal with Vertex extends Editas Medicine’s cash runway into 2026.
The company’s stock gained 5.7% in the last trading session as the investors cheered the encouraging collaboration agreement. Year to date, shares of Editas have gained 17.2% against the industry’s 19.2% decline.
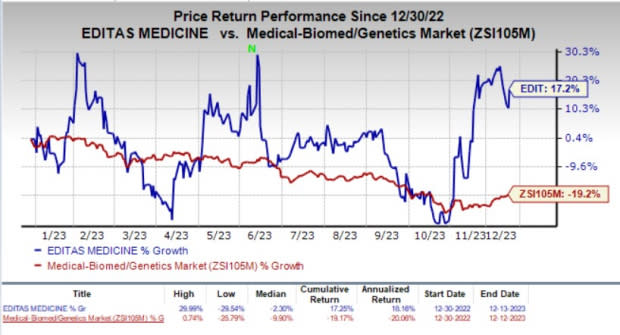
Image Source: Zacks Investment Research
The company reported holding exclusive licenses to certain CRISPR patent estates for making human medicines, which include a Cas9 patent estate.
Editas is currently using its CRISPR/Cas12a and CRISPR/Cas9 genome editing systems to develop its pipeline candidates for treatments for people living with serious diseases.
The company is focused on the development of its lead candidate, EDIT 301,now known as renizgamglogene autogedtemcel (reni-cel), as a potential one-time, durable gene editing medicine to treat SCD and transfusion-dependent beta thalassemia (TDT). The candidate is currently being developed in two separate early-mid-stage studies, RUBY and EdiTHAL, to treat SCD and TDT, respectively.
Earlier this week, the company reported new positive safety and efficacy data in 17 patients treated with reni-cel in the RUBY and EdiTHAL studies (11 and six patients, respectively). It was observed that the candidate was overall well tolerated. The investigational drug demonstrated a consistent safety profile in both studies.
In terms of efficacy, it was observed that all patients treated with reni-cel in the RUBY study were free of vaso-occlusive events since the start of the treatment. In the EdiTHAL study, it was observed that all patients treated with reni-cel experienced an early and robust increase of total hemoglobin, above the transfusion independence threshold of 9 g/dl.
Editas Medicine, Inc. Price and Consensus
Editas Medicine, Inc. price-consensus-chart | Editas Medicine, Inc. Quote
Last week, the FDA achieved a historic milestone when it approved two one-shot cell-based gene therapies, namely Casgevy and Lyfgenia (lovo-cel), for treating SCD in patients aged 12 years and older.
Lyfgenia has been developed by bluebird bio BLUE, while Casgevy has been jointly developed by CRISPR Therapeutics CRSP and Vertex.
Among the two gene therapies, the approval of CRISPR Therapeutics/Vertex’s Casgevy was more impressive since it marks the first time that the FDA approved a gene therapy utilizing the Nobel prize-winning CRISPR technology. Per the FDA, the Casgevy approval marks an important “innovative advancement” in the world of gene therapies. Lyfgenia uses a lentiviral vector (gene delivery vehicle) for genetic modifications.
The FDA approvals were based on positive data from the companies’ clinical studies, wherein treatment with both therapies has helped reduce painful episodes in SCD patients.
The gene therapy drugs, however, come with a hefty price tag. Vertex and CRISPR Therapeutics disclosed that they would commercially launch Casgevy at $2.1 million. In a separate press release, bluebird bio announced that it will launch Lyfgenia at $3.1 million.
It is also important to note that treatment with either the CRISPR Therapeutics/Vertex or bluebird bio gene therapies has its fair share of side effects, which include low levels of platelets, white blood cells and fertility problems.
bluebird bio‘s Lyfgenia label includes a black box warning for hematologic malignancy (blood cancer). Patients infused with bluebird bio’s therapy are required to be monitored for this malignancy for life.
Zacks Rank
Editas currently has a Zacks Rank #3 (Hold).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Vertex Pharmaceuticals Incorporated (VRTX) : Free Stock Analysis Report
bluebird bio, Inc. (BLUE) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
