Editas (EDIT) Posts Upbeat Initial Data From SCD & TDT Studies
Editas Medicine EDIT announced positive preliminary safety and efficacy data from the first four patients with sickle cell disease (SCD) treated with EDIT-301 in the RUBY study and the first transfusion-dependent beta thalassemia (TDT) patient treated in the EdiTHAL study.
Per Editas, the first and second patients in the RUBY study achieved normal hemoglobin levels five months post-treatment with EDIT-301, maintaining a normal hemoglobin level at 10 and six months of follow up, respectively. Furthermore, both patients were observed to have maintained consistent fetal hemoglobin levels of greater than 40% during the same time frame.
The third and fourth patients, in the RUBY study, demonstrated an increase in total hemoglobin and fetal hemoglobin at three and two months of follow up, respectively, thereafter showing similar trends as those observed in the first two patients. Furthermore, Editas reported that all four treated RUBY patients are free of vaso-occlusive events since the infusion of EDIT-301.
Editas is also evaluating EDIT-301 for the treatment of TDT. The company dosed the first patient in the phase I/II EdiTHAL study of EDIT-301, for treating TDT, in the first quarter of 2023. The patient reportedly had successful neutrophil and platelet engraftment. Editas reported that the patient’s response to treatment with EDIT-301 resembled that of the first four RUBY patients at one and a half months post-infusion.
So far this year, shares of Editas have gained 9% against the industry’s 7.8% fall.
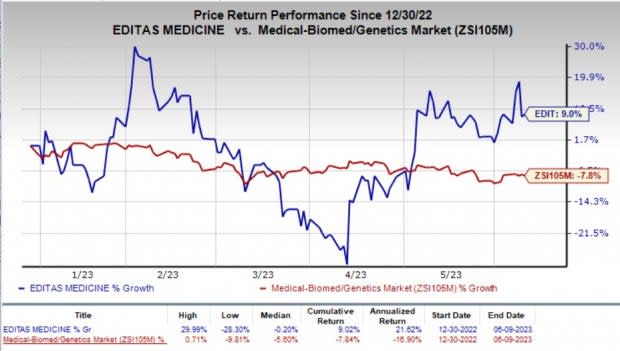
Image Source: Zacks Investment Research
Per observation, treatment with EDIT-301 was well-tolerated by all four patients in the RUBY study as well as the first patient in the EdiTHAL study. Treatment with EDIT-301 also demonstrated a consistent safety profile. Furthermore, no serious adverse events occurred upon treatment with EDIT-301 and none of the adverse events reported were related to treatment with EDIT-301.
Editas remains on track to dose 20 patients in the RUBY study along with additional updates from the same study by the end of 2023. Top-line data from the EdiTHAL study is also expected by the year’s end.
In April 2023, the FDA granted EDIT-301, the orphan drug designation, for the treatment of SCD. Previously, EDIT-301 received the FDA’s Orphan Drug Designation for the treatment of beta thalassemia and Rare Pediatric Disease designation to EDIT-301 for the treatment of beta thalassemia and sickle cell disease.
The FDA’s orphan drug designation for both these indications will grant EDIT-301 market exclusivity in the United States, along with a few other perks, subject to approval.
Editas Medicine, Inc. Price and Consensus
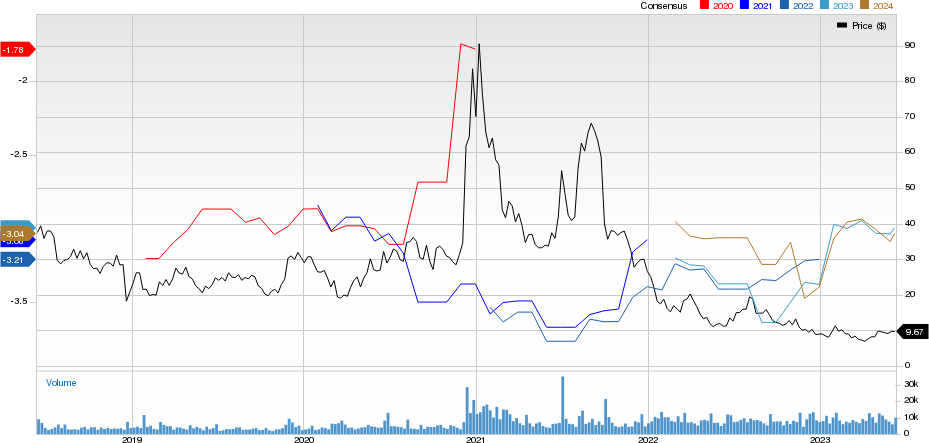
Editas Medicine, Inc. price-consensus-chart | Editas Medicine, Inc. Quote
Zacks Rank and Stocks to Consider
Editas Medicine currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Adaptimmune Therapeutics ADAP, Akero Therapeutics AKRO and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has remained stable at 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from 74 cents to 56 cents. In the year so far, shares of Adaptimmune Therapeutics have fallen by 32.4%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $3.46 to $2.80. During the same period, the estimate for Akero Therapeutics’ 2024 loss per share has narrowed from $3.66 to $3.32. In the year so far, shares of Akero Therapeutics have fallen by 4.2%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 19 cents to 9 cents. The consensus estimate for ADMA Biologics’ 2024 earnings is currently pegged at 7 cents per share. In the year so far, shares of ADMA Biologics have gained 1.5%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
