Editas' (EDIT) Q2 Earnings Beat, Sales Miss, Pipeline in Focus
Editas Medicine, Inc. EDIT incurred a loss of 56 cents per share in the second quarter of 2023, narrower than the Zacks Consensus Estimate of a loss of 76 cents. The company had reported a loss of 78 cents per share in the year-ago quarter.
Collaboration and other research and development revenues, which comprise the company’s top line, were $2.9 million in the reported quarter, down 54.7% from $6.4 million reported in the year-ago quarter. The reported figure missed the Zacks Consensus Estimate of $6 million. Per management, the decrease in collaboration revenues was on account of Bristol Myers’ BMY program opt-in in the second quarter of 2022 which did not occur in the same period of 2023.
Editas is advancing alpha-beta T-cell experimental medicines for the treatment of solid and liquid tumors in collaboration with Bristol Myers through its wholly-owned subsidiary, Juno Therapeutics, Inc. The company also has an existing collaboration agreement with Shoreline Biosciences for its SLEEK gene editing knock-in technology along with iNK cell programs.
EDIT’s stock jumped about 6% on Wednesday owing to the earnings beat as well as its encouraging pipeline progress during the second quarter of 2023. Year to date, shares of Editas have gained 3% against the industry’s 12.3% decline.
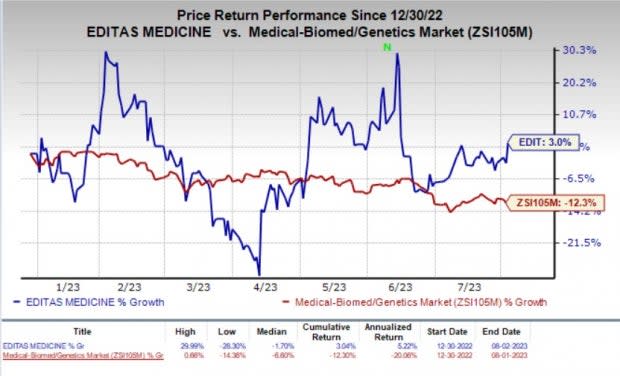
Image Source: Zacks Investment Research
Quarter in Detail
In the second quarter of 2023, research and development expenses (R&D) decreased to $29.8 million compared with $43.7 million in the year-ago period. The 31.8% decline in R&D expenses was due to Editas’ strategic reprioritization, including a targeted clinical and manufacturing focus on EDIT-301, and reduced employee-related costs.
Under the strategic reprioritization effort announced in January 2023, Editas redirected all its resources to the development of EDIT-301 as its lead candidate. The company also discontinued internal investments in inherited retinal disease programs and its wholly owned multiplexed edited iPSC-derived iNK cell programs, only to be developed in collaboration. The workforce of the company has also been reduced by approximately 20% to extend the cash runway into 2025.
General and administrative expenses were $17.2 million in the reported quarter, which remained flat year over year. The slight improvement was on the grounds of increased professional services expenses to support business development activities, partially offset by a decrease in stock compensation expense.
Editas had cash, cash equivalents and investments worth $480 million as of Jun 30, 2023 compared with $401.8 million as of Mar 31, 2023. The company expects its existing cash, cash equivalents and marketable securities to fund operating expenses and capital expenditure into the third quarter of 2025.
Pipeline Updates
Editas has no approved products in its portfolio at the moment. Therefore, pipeline development remains the key focus of the company.
The company is evaluating the safety and efficacy of its investigational gene-editing medicine, EDIT-301, for treating sickle cell disease (SCD). Editas remains on track to dose 20 total SCD patients in the RUBY study of EDIT-301 by year-end.
EDIT is also on track to provide an additional clinical update from the RUBY study by year-end. EDIT-301 enjoys the FDA’s Orphan Drug Designation for the treatment of SCD.
Editas is also evaluating EDIT-301 for the treatment of transfusion-dependent beta thalassemia (TDT). The company commenced parallel dosing in the phase I/II EDITHAL study in the second quarter of 2023. Editas is on track to provide an additional clinical update from the EDITHAL study by the end of the year.
During the quarter, Editas announced positive preliminary safety and efficacy data from the first four patients with SCD treated with EDIT-301 in the RUBY study and the first TDT patient treated in the EDITHAL study.
Editas Medicine, Inc. Price, Consensus and EPS Surprise
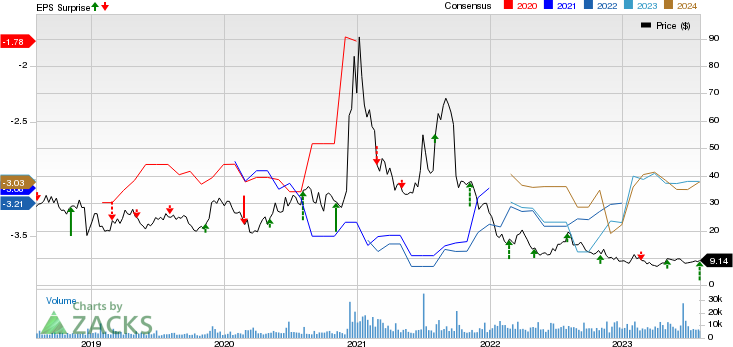
Editas Medicine, Inc. price-consensus-eps-surprise-chart | Editas Medicine, Inc. Quote
Zacks Rank and Stocks to Consider
Editas currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the overall medical sector are J&J JNJ and ADC Therapeutics ADCT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for J&J’s 2023 earnings per share has increased from $10.66 to $10.73. During the same period, the estimate for JNJ’s 2024 earnings per share has increased from $11.01 to $11.28. Year to date, shares of JNJ have lost 3.8%.
JNJ beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.58%.
In the past 90 days, the Zacks Consensus Estimate for ADC Therapeutics’ 2023 loss per share has widened from $2.60 to $2.61. During the same period, the estimate for ADC Therapeutics’ 2024 loss per share narrowed from $2.75 to $2.55. Year to date, shares of ADCT have lost 61.2%.
ADCT beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 10.70%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
ADC Therapeutics SA (ADCT) : Free Stock Analysis Report
Editas Medicine, Inc. (EDIT) : Free Stock Analysis Report
