Enlivex Presented Substantial Survival Benefit of Allocetra in Combination with Immune Checkpoint Inhibitor in Mesothelioma Cancer Study at the International Society for Cell and Gene Therapy Annual 2022 Meeting

chart - click to enlarge

Mesothelioma is one of the deadliest solid cancers, with few treatment options, all of which have limited efficacy. Immune checkpoint inhibitors targeting CTLA4 and PD1 are FDA-approved as a first-line treatments for patients with unresectable malignant pleural mesothelioma
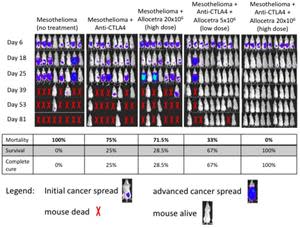
Up to 100% survival in the combination therapy group (AllocetraTM + anti-CTLA4 immune checkpoint inhibitor) of a preclinical mesothelioma mice study vs. 0% survival in the untreated group and up to 25% survival in the group treated with the stand-alone commercially approved anti-CTLA4. Stand-alone AllocetraTM treatment was comparable to anti-CTLA4 (28.5% survival)
Results position AllocetraTM as a highly-differentiated cell therapy for the treatment of difficult-to-treat solid tumors in combination with immune checkpoint inhibitor therapies
Nes-Ziona, Israel, May 09, 2022 (GLOBE NEWSWIRE) -- Enlivex Therapeutics Ltd. (Nasdaq: ENLV, the “Company”), a clinical-stage macrophage reprogramming immunotherapy company, announced the poster presentation of a substantial survival benefit of AllocetraTM combined with immune checkpoint inhibition in a preclinical mesothelioma study at the International Society of Cell and Gene Therapy Annual 2022 Meeting. The poster was titled, “Allocetra-OTS, an Early Apoptotic Cellular Therapy Synergize with Chimeric Antigen Receptor (CAR) T Cell Therapy or Immune Check Point Inhibitor Against Peritoneal Solid Tumor.”
Mesothelioma, Treatment Landscape, and Macrophage-Solid Tumor Dynamics
Mesothelioma is one of the deadliest solid cancers with few treatment options, all of which have limited efficacy. People who are most at risk to develop mesothelioma generally have had long-term exposure to asbestos (e.g., construction workers, pipe fitters, and shipyard workers). It takes many years for mesothelioma to develop; it can appear 30 years after asbestos exposure. Immune checkpoint inhibitors targeting CTLA4 and PD1 are FDA-approved as first-line treatments for patients with unresectable malignant pleural mesothelioma.
In mesothelioma and in some other solid cancers, tumor cells, as part of their defense mechanisms, facilitate the recruitment of macrophages which become “pro-tumor” tumor associated macrophages (TAMs) rather than “anti-tumor” macrophages. The TAMs typically form a physical layer on top of the solid tumor, and induce immunosuppression in the solid tumor microenvironment. This in-turn promotes tumor growth and metastasis and makes it very difficult for the immune system or any anti-cancer drug to efficiently attack the cancerous cells. Allocetra™ is a cell therapy in development that is targeted at these TAMs, and its proposed mechanism of action is to change the balance of macrophage populations to skew towards anti-tumor macrophages and away from pro-tumor macrophages.
The Mesothelioma Model
To test the potential effect of cell therapy-induced macrophage reprogramming on difficult-to-treat solid tumors, Enlivex ran preclinical studies in which five groups of mice were implanted with mesothelioma cancer cells. The difference between the groups was the treatment given, which started on day 12 after the cancer’s initial growth period.
Results from multiple studies strongly support the potential of AllocetraTM to assist in the process of macrophage reprogramming in the solid tumor microenvironment and significantly change survival outcomes. As expected, only 1/16 (6%) of the untreated mice remained alive at day 42 (that one last remaining mouse died on day 65), compared with up to 25% survival for the mice in the group that was treated with the anti-CTLA4 immune checkpoint inhibitor. Surprisingly, AllocetraTM as a stand-alone treatment provided comparable survival rates as anti-CTLA4 monotherapy, with a 28.5% survival rate and a delayed cancer growth rate observed. Notably, the synergistic effect of treating with both drugs resulted in up to 100% survival with complete disappearance of the cancer. The effect was correlated with the AllocetraTM treatment dose. A lower dose of AllocetraTM provided slightly lower survival and cancer complete remission rates (67% complete remission for the low-dose treatment vs. up to 100% for the higher dose). The attached chart provides a visual guide to the progressions/regression of the mesothelioma cancer in the different groups, as recorded for study mesothelioma AB12 137.
Across multiple studies of the AB12 mesothelioma model, anti-CTLA4 therapy significantly improved survival duration from mean 34±9 to 44.9 ±20 days (p<0.05). Allocetra™ as a stand-alone therapy improved survival duration to 52.3 ±20 days (p<0.02). The synergistic effect of the combination therapy of anti-CTLA4 + AllocetraTM improved survival duration to 86.7±20 days (p<0.0001) with complete cancer remission in 60-100% of mice, depending on the dose administered.
Prof. Dror Mevorach, Chief Scientific Officer of Enlivex, stated: “This reproducible and statistically significant data strongly support Allocetra’s proposed therapeutic mechanism of action of reprogramming macrophages in solid tumors. We believe that, in contrast to CAR-T, CAR-NK and other anti-cancer cell therapies directed at tumor antigens, AllocetraTM restores macrophage homeostasis in the tumor environment via reprogramming of pro-tumor macrophages. This may ultimately allow immune checkpoint inhibitors to be exponentially more effective.”
Oren Hershkovitz, Ph.D., CEO of Enlivex, added: “Based on these results, Enlivex is planning to initiate a series of clinical trials during 2022 that are designed to evaluate Allocetra™ in combination with FDA-approved immune checkpoint inhibitor therapies in patients with advanced-stage solid tumors. To date, we have infused AllocetraTM in dozens of hospitalized patients in fragile condition resulting from sepsis and COVID-19-related organ dysfunction. We believe that the proposed differentiated mechanism of action of AllocetraTM, together with the safety and tolerability it has demonstrated in these prior studies, as well as the encouraging efficacy results of our preclinical solid tumor models, highlight an intriguing opportunity for Enlivex to provide another layer of life-saving therapy for patients who have few available options.”
ABOUT ALLOCETRA™
Allocetra™ is being developed as a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Diseases such as solid cancers, sepsis, and many others reprogram macrophages out of their homeostatic state. These non-homeostatic macrophages contribute significantly to the severity of the respective diseases. By restoring macrophage homeostasis, Allocetra™ has the potential to provide a novel immunotherapeutic mechanism of action for life-threatening clinical indications that are defined as "unmet medical needs", as a stand-alone therapy or in combination with leading therapeutic agents.
ABOUT ENLIVEX
Enlivex is a clinical-stage macrophage reprogramming immunotherapy company developing Allocetra™, a universal, off-the-shelf cell therapy designed to reprogram macrophages into their homeostatic state. Resetting non-homeostatic macrophages into their homeostatic state is critical for immune system rebalancing and resolution of life-threatening conditions. For more information, visit http://www.enlivex.com.
Safe Harbor Statement: This press release contains forward-looking statements, which may be identified by words such as “expects,” “plans,” “projects,” “will,” “may,” “anticipates,” “believes,” “should,” “would”, “could,” “intends,” “estimates,” “suggests,” “has the potential to” and other words of similar meaning, including statements regarding expected cash balances, market opportunities for the results of current clinical studies and preclinical experiments, the effectiveness of, and market opportunities for, ALLOCETRATM programs. All such forward-looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Investors are cautioned that forward-looking statements involve risks and uncertainties that may affect Enlivex’s business and prospects, including the risks that Enlivex may not succeed in generating any revenues or developing any commercial products; that the products in development may fail, may not achieve the expected results or effectiveness and/or may not generate data that would support the approval or marketing of these products for the indications being studied or for other indications; that ongoing studies may not continue to show substantial or any activity; and other risks and uncertainties that may cause results to differ materially from those set forth in the forward-looking statements. The results of clinical trials in humans may produce results that differ significantly from the results of clinical and other trials in animals. The results of early-stage trials may differ significantly from the results of more developed, later-stage trials. The development of any products using the ALLOCETRATM product line could also be affected by a number of other factors, including unexpected safety, efficacy or manufacturing issues, additional time requirements for data analyses and decision making, the impact of pharmaceutical industry regulation, the impact of competitive products and pricing and the impact of patents and other proprietary rights held by competitors and other third parties. In addition to the risk factors described above, investors should consider the economic, competitive, governmental, technological and other factors discussed in Enlivex’s filings with the Securities and Exchange Commission, including in the Company’s most recent Annual Report on Form 20-F filed with the Securities and Exchange Commission. The forward-looking statements contained in this press release speak only as of the date the statements were made, and we do not undertake any obligation to update forward-looking statements, except as required under applicable law.
ENLIVEX CONTACT
Shachar Shlosberger, CFO
Enlivex Therapeutics, Ltd.
shachar@enlivexpharm.com
INVESTOR RELATIONS CONTACT
Eric Ribner
LifeSci Advisors
eric@lifesciadvisors.com
Attachment

