Entrada Therapeutics Inc (TRDA) Reports Solid Financial Position and Advancements in Clinical Trials
Cash Position: Increased to $352 million as of December 31, 2023, extending the cash runway through Q2 2026.
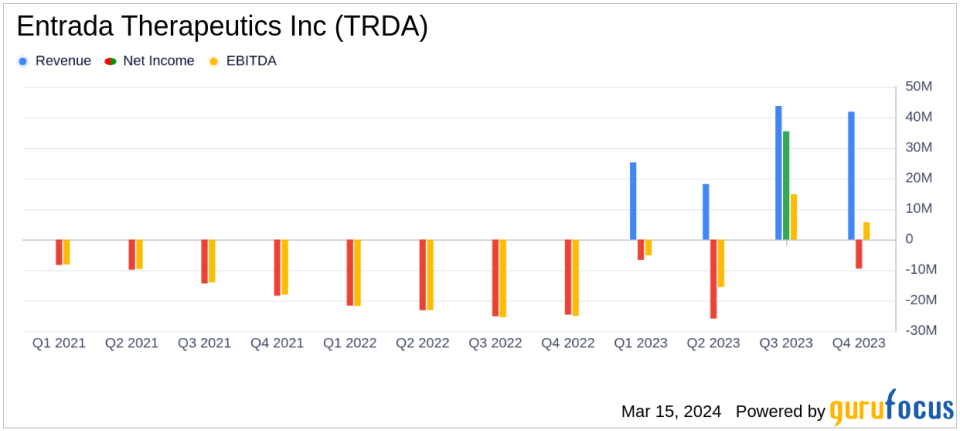
Collaboration Revenue: Reported $41.8 million for Q4 and $129.0 million for the full year of 2023.
R&D Expenses: Grew to $28.3 million in Q4 and $99.9 million for the full year, reflecting investment in clinical trials and platform expansion.
G&A Expenses: Totaled $8.7 million in Q4, with a full-year expense of $32.3 million, mainly due to increased personnel costs.
Net Loss: Decreased to $9.5 million in Q4 and $6.7 million for the full year, significantly improving from 2022 figures.
Entrada Therapeutics Inc (NASDAQ:TRDA) released its 8-K filing on March 13, 2024, detailing its financial results for the fourth quarter and full year ending December 31, 2023. The company, a clinical-stage biopharmaceutical entity, is committed to pioneering a new class of intracellular medicines with its Endosomal Escape Vehicle (EEV)-therapeutics, targeting diseases that have been challenging to treat due to previously inaccessible intracellular targets.
Financial Highlights and Clinical Advancements
Entrada Therapeutics reported a robust cash position of $352 million, a significant increase from the previous year's $188.7 million. This financial strength is attributed to the proceeds from the Vertex Agreement and is expected to support the company's operations and capital expenditure requirements through the second quarter of 2026.
The company's collaboration revenue saw a remarkable rise to $41.8 million in the fourth quarter and $129.0 million for the full year of 2023, a noteworthy achievement considering there was no collaboration revenue in the previous year. This surge in revenue is crucial for Entrada Therapeutics as it underscores the value of its strategic partnerships and the potential of its therapeutic pipeline.
Research and Development (R&D) expenses increased to $28.3 million for the fourth quarter and $99.9 million for the full year, up from $15.7 million and $66.6 million in the same periods of 2022, respectively. This increase reflects the company's commitment to advancing its clinical trials, particularly the ENTR-601-44 Phase 1 trial, and its investment in future trials and platform expansion. The rise in R&D expenses is a vital indicator of the company's dedication to innovation and progress in the biotechnology industry.
General and Administrative (G&A) expenses were reported at $8.7 million for the fourth quarter, with a slight increase in the full year to $32.3 million. This was primarily due to higher personnel costs, including non-cash stock-based compensation, which is common as companies in this sector scale up their operations.
The net loss for the fourth quarter was $9.5 million, and $6.7 million for the full year, showing a significant improvement from the net loss of $24.6 million and $94.6 million for the same periods in 2022, respectively. This reduction in net loss is an encouraging sign for investors, as it may indicate a trajectory towards profitability.
Strategic Corporate Developments
Entrada Therapeutics has made notable progress in its clinical trials, completing dosing for the first three cohorts of its Phase 1 clinical trial, ENTR-601-44-101, for the potential treatment of Duchenne muscular dystrophy (DMD). The company anticipates announcing data from this trial in the second half of 2024 and expects to submit regulatory applications for global Phase 2 clinical development of ENTR-601-44 and ENTR-601-45 in the fourth quarter of 2024.
Additionally, Vertex Pharmaceuticals has initiated a Phase 1/2 clinical trial of VX-670 for patients with myotonic dystrophy type 1 (DM1), further highlighting Entrada's collaborative efforts.
The company has also strengthened its leadership team with strategic appointments, positioning itself for continued growth and success in the biopharmaceutical industry.
Looking Ahead
Entrada Therapeutics' financial results and clinical advancements in 2023 set a positive tone for the company's future. With a solid cash position, increased collaboration revenue, and strategic investments in R&D, Entrada is poised to continue its mission of bringing innovative treatments to patients with serious and rare diseases. The anticipated data readouts and regulatory submissions in 2024 could be pivotal for the company's growth and the advancement of its therapeutic pipeline.
For more detailed information on Entrada Therapeutics Inc (NASDAQ:TRDA)'s financial results and corporate updates, investors and interested parties are encouraged to review the full 8-K filing.
Explore the complete 8-K earnings release (here) from Entrada Therapeutics Inc for further details.
This article first appeared on GuruFocus.
