ETON Up as FDA Accepts NDA for Dehydrated Alcohol Injection
Shares of Eton Pharmaceuticals, Inc. ETON surged 14.91% after it announced that the FDA has accepted to review its new drug application (NDA) response for dehydrated alcohol injection for the proposed indication of methanol poisoning. The regulatory body has set a target action date of Jun 27, 2023.
The product was previously granted orphan drug designation for the indication of methanol poisoning. Upon a potential FDA approval, the regulatory body is expected to grant the application seven years of orphan drug exclusivity. Based on IQVIA data, trailing 12-month sales for dehydrated alcohol injection were $74 million.
Shares of the company have lost 11% in the past year compared with the industry’s decrease of 13.8%.
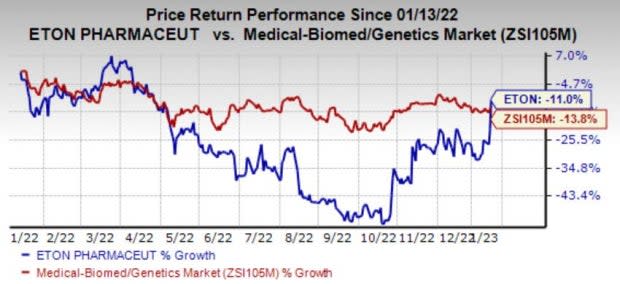
Image Source: Zacks Investment Research
A tentative approval will broaden the company’s portfolio. Please note that Eton currently has three commercial products for rare diseases, namely, Alkindi Sprinkle for the treatment of adrenocortical insufficiency, Carglumic Acid for the treatment of acute hyperammonemia due to N-acetylglutamate synthase (NAGS) deficiency and Betaine Anhydrous for the treatment of homocystinuria and has three additional product candidates in late-stage development.
Eton acquired Betaine Anhydrous in the third quarter and the product shares the same metabolic geneticist prescriber base as Carglumic Acid and is expected to be accretive to the bottom line in 2023.
Eton Pharmaceuticals, Inc. Price and Consensus
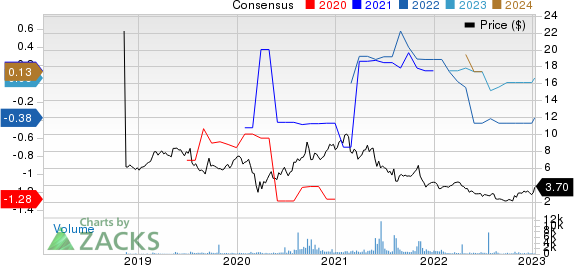
Eton Pharmaceuticals, Inc. price-consensus-chart | Eton Pharmaceuticals, Inc. Quote
In addition, Eton is entitled to royalties or milestone payments from six FDA-approved products that the company developed and out-licensed. The products are Alaway Preservative Free, EPRONTIA, Cysteine Hydrochloride, Zonisade, Biorphen, and Rezipres.
Zonisade was developed as part of Eton’s multi-product neurology oral solution agreement with Azurity Pharmaceuticals. Azurity is solely responsible for commercializing the product; Eton is entitled to receive milestone payments and royalties on net sales of the product. Zonisade was launched in October, triggering a $5 million milestone payment to Eton that was recognized in the fourth quarter.
Zacks Rank & Other Stocks to Consider
Eton currently sports a Zacks Rank #1 (Strong Buy). Some other top-ranked stocks in the biotech sector are Syndax Pharmaceuticals, Inc. SNDX and Inovio Pharmaceuticals INO. While Syndax carries the same rank as Eton, INO carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss per share estimates for Syndax Pharmaceuticals for 2022 and 2023 have narrowed by 11 cents and 20 cents, respectively, over the past 60 days. Earnings of Syndax Pharmaceuticals surpassed estimates in three of the trailing four quarters and met the same on the other occasion. SNDX witnessed an earnings surprise of 95.39% on average.
Loss per share estimates for INO have narrowed by 29 cents and 36 cents for 2022 and 2023, respectively, in the past 60 days.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Inovio Pharmaceuticals, Inc. (INO) : Free Stock Analysis Report
Syndax Pharmaceuticals, Inc. (SNDX) : Free Stock Analysis Report
Eton Pharmaceuticals, Inc. (ETON) : Free Stock Analysis Report
