Exelixis (EXEL) Posts Preliminary 2023 Results, to Cut 13% Jobs
Exelixis EXEL announced preliminary financial results for 2023, the annual outlook for 2024 and other corporate updates.
Revenues for 2023 totaled around $1.83 billion, while product revenues came in at $1.63 million. Research and development expenses amounted to approximately $1.045 billion and selling, general and administrative expenses totaled $545 million.
The Zacks Consensus Estimate for revenues in 2023 was $1.84 billion.
For 2024, Exelixis expects revenues to be between $1.825 billion and $1.925 billion, while product revenues are projected to be in the $1.65-$1.75 billion range. Research and development expenses are estimated to be in the range of $925-$975 million. Selling, general and administrative expenses are projected in the band of $425-$475 million.
Management will focus on the label expansion of its lead drug Cabometyx in 2024 and accelerate the development of zanzalintinib, XB002 and XL309, and advance three promising preclinical programs into clinical development. Cabometyx (cabozantinib tablets) is approved for advanced renal cell carcinoma (“RCC”) and previously treated hepatocellular carcinoma.
Management will present detailed data from the phase III CONTACT-02 study, evaluating the combination of cabozantinib and Tecentriq (atezolizumab) versus a second novel hormonal therapy (“NHT”) in patients with metastatic castration-resistant prostate cancer (“mCRPC”) and measurable extrapelvic soft tissue disease who have been previously treated with one NHT.
The trial met one of its primary endpoints of progression-free survival (as announced in August 2023). The study continues with the analysis of the second primary endpoint of overall survival, which is anticipated in 2024. Exelixis will continue its discussions with the FDA on a potential regulatory path forward for cabozantinib in mCRPC.
A potential regulatory filing for cabozantinib in advanced neuroendocrine tumors (“NET”) based on positive results from the pivotal phase III CABINET study, which evaluates cabozantinib versus placebo in patients with either advanced pancreatic NET or extra-pancreatic NET, is also targeted in 2024.
Zanzalintinib, a third-generation tyrosine kinase inhibitor (“TKI”), is being evaluated in three ongoing pivotal trials, STELLAR-303, -304 and -305, in forms of colorectal cancer, non-clear cell RCC and squamous cell carcinoma of the head and neck, respectively.
XB002 is a next-generation tissue factor-targeting antibody-drug conjugate (“ADC”) that is being evaluated as a monotherapy and in combination regimens. This year, Exelixis is focused on advancing JEWEL-101, the phase I study of XB002 alone and in combination with immunotherapy in a variety of solid tumor settings, with the goal of prioritizing sensitive tumor types for full development.
XL309, a potentially best-in-class small-molecule inhibitor of USP1, has emerged as a synthetic lethal target in the context of BRCA-mutated tumors. Exelixis in-licensed XL309 from Insilico Medicine in September 2023 and took responsibility for the ongoing phase I study in the fourth quarter of 2023.
Exelixis’ clinical development priorities for XL309 include accelerating its development as a potential therapy for tumors that have become refractory to PARP inhibitor (“PARPi”) therapy, including forms of ovarian, breast and prostate cancers, pursuing potential PARPi combinations and moving beyond the PARPi market into new areas.
Shares of the company have risen 21.9% in the past six months compared with the industry’s growth of 0.3%.
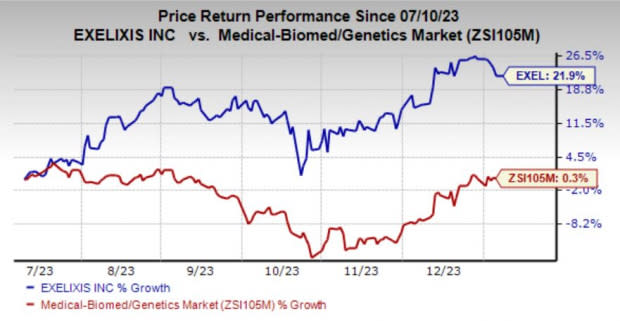
Image Source: Zacks Investment Research
Concurrently, the company announced the implementation of corporate restructuring that will prioritize the advancement of its deep pipeline of clinical and near-clinical programs. As a result, EXEL will reduce its headcount by approximately 175 employees or 13%. The firm expects to substantially complete the restructuring in the first quarter of 2024 and incur a restructuring charge of approximately $25 million.
Separately, its board of directors authorized the repurchase of up to an additional $450 million of the company’s common stock in 2024. Exelixis completed its previously announced repurchase of 26.2 million shares of its common stock or 8% of shares outstanding, for a total of $550 million in 2023.
EXEL plans to file an investigational new drug (“IND”) application for the XB010 5T4-MMAE ADC program in the first half of 2024 and expects to file IND applications for the XB628 PD-L1-NKG2A bispecific antibody and XL495 small molecule PKMYT1 inhibitor programs in the second half of 2024 if preclinical data continues to remain supportive.
EXEL is striving to expand Cabometyx’s label and concurrently develop its portfolio with promising candidates zanzalintnib, XB002 and XL309.
Cabometyx maintained its status as the leading TKI in the third quarter for the treatment of RCC. This was driven by its use in combination with Bristol Myers’ BMY Opdivo in the first-line setting.
The drug also maintained growth in the hepatocellular carcinoma.
Merck’s Keytruda and BMY’s Opdivo are leading immuno-oncology drugs approved for various oncology indications.
The successful development of additional drugs will broaden its portfolio and reduce its dependence on its lead drug, Cabometyx.
However, capturing additional market share in the RCC space will be a daunting task for Exelixis, given the competition.
Zacks Rank & Stocks to Consider
Exelixis currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Entrada Therapeutics TRDA and Acadia Pharmaceuticals ACAD. While TRDA sports a Zacks Rank #1 (Strong Buy), ACAD has a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Entrada’s loss per share estimate for 2023 has narrowed from $2.07 to 9 cents in the past 60 days. The same for 2024 has narrowed from $2.35 to $2.04 during the same time frame.
Loss per share estimate for Acadia has narrowed to 33 cents from 41 cents for 2023. The bottom-line estimate for 2024 is currently pinned at $1.04. ACADIA's shares have risen 22.5% in the past six months.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Entrada Therapeutics, Inc. (TRDA) : Free Stock Analysis Report
