Exelixis (EXEL) Stock Gains 41.8% So Far in 2023: Here's Why
Exelixis, Inc. EXEL is having a phenomenal year so far, with its shares gaining 41.8% against the industry’s decline of 11.9%.
Exelixis’ second-quarter results were better than expected on the back of strong demand for lead drug, Cabometyx. Cabozantinib tablets are approved in the United States under the brand name, Cabometyx, for the treatment of patients with advanced renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC) who have been previously treated with sorafenib, and for patients with advanced RCC as a first-line treatment in combination with Bristol Myers’ BMY Opdivo.
Cabometyx maintained its status as the leading tyrosine kinase inhibitor (TKI) in the second quarter for the treatment of RCC, driven by its use in combination with Opdivo in the first-line setting and second-line monotherapy setting for the HCC indication.
Per the company, Cabometyx, in combination with Opdivo, remains the number one TKI plus immuno-oncology combination in first-line RCC.
Bristol-Myers’ Opdivo is one of the leading revenue generators for the company and is approved for various oncology indications.
In addition, the pipeline progress has been impressive as the company strives to expand Cabometyx’s label and concurrently develop its portfolio beyond its lead drug.
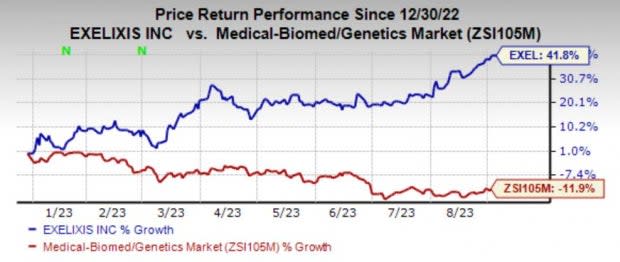
Image Source: Zacks Investment Research
Last month, the company and partner Ipsen announced that the late-stage CONTACT-02 study achieved one of the two primary endpoints. The study is evaluating cabozantinib in combination with Tecentriq (atezolizumab) for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC) who have a measurable visceral disease or measurable extra pelvic adenopathy and have been previously treated with one novel hormonal therapy. Data from the study showed cabozantinib in combination with Tecentriq demonstrated a statistically significant reduction in the risk of disease progression or death compared with a second novel hormonal therapy in mCRPC patients.
In addition, a trend toward improved overall survival (OS) was observed at a prespecified interim analysis. However, the data was immature and did not meet the threshold for statistical significance. Therefore, the trial will proceed to the next OS analysis as planned.
Exelixis also announced positive results from the late-stage CABINET study evaluating cabozantinib in advanced pancreatic neuroendocrine tumors or advanced extra-pancreatic neuroendocrine tumors (also referred to as carcinoid tumors) in patients who have experienced progression after prior systemic therapy. Data showed cabozantinib substantially prolonged the time without disease progression or death in both of the trial’s cohorts.
In July, Exelixis announced that it entered into a settlement and license agreement with Teva Pharmaceuticals TEVA. This settlement resolves patent litigation brought by Exelixis in response to Teva’s abbreviated new drug application seeking approval to market a generic version of Cabometyx prior to the expiration of the applicable patents. Per the settlement terms, Exelixis will grant Teva a license to market its generic version of the drug in the United States beginning on Jan 1, 2031, upon the FDA’s approval. Consequently, both companies will terminate the ongoing litigation. This is a definite positive for Exelixis.
Shares also soared in March after Exelixis announced a share repurchase program of $550 million. The program will be completed before the end of 2023. Investors cheered the company’s effort to return shareholders' value through this repurchase program.
Meanwhile, Exelixis is striving hard to develop its portfolio beyond Cabometyx with promising candidates - zanzalintnib, XB002, XL102 and CBX-12.
Exelixis completed enrollment in multiple expansion cohorts of the phase I STELLAR-001 study for zanzalintinib and progressed to the ongoing phase III studies.
The company also continued to advance the phase I JEWEL-101 study for XB002, selecting the single-agent dose from the dose-escalation stage of the study and initiating the cohort expansion stage, with the goal of moving the program into full development before year’s end.
The successful development of additional drugs will broaden its portfolio and reduce its dependence on the lead drug, Cabometyx.
However, capturing additional market share in the RCC will be a daunting task for Exelixis, given the competition.
Exelixis currently carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Teva Pharmaceutical Industries Ltd. (TEVA) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
