Fate Therapeutics (FATE) Q4 Earnings Beat, Revenues Fall Y/Y
Fate Therapeutics FATE reported fourth-quarter 2023 loss of 45 cents per share, narrower than the Zacks Consensus Estimate of a loss of 57 cents and the year-ago loss of 58 cents.
The loss narrowed year over year due to lower research and development (R&D) expenses.
The company earned collaboration revenues of $2 million, which also surpassed the Zacks Consensus Estimate of $1 million but was down from $44 million reported in the year-ago quarter.
Revenues were derived from the company’s preclinical development activities for a second collaboration candidate targeting an undisclosed solid tumor antigen under its collaboration with ONO Pharmaceutical.
R&D expenses declined 64% to $31.8 million in the reported quarter. General and administrative expenses also declined 17% to $17.9 million. The lower operating expenses were due to a decrease in salaries and benefits, including share-based compensation expenses following the company's restructuring in the first quarter of 2023. R&D expenses also declined due to lower clinical study costs and demand for R&D materials and equipment.
Cash, cash equivalents and investments as of Dec 31, 2023, were $316.2 million compared with $349.7 million as of Sep 30, 2023.
Shares of FATE have risen 13.6% in the past year against the industry’s decline of 7.2%.
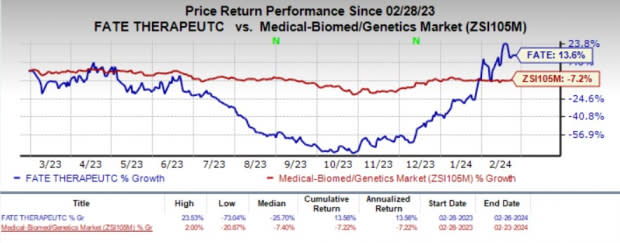
Image Source: Zacks Investment Research
Full-Year 2023 Results
Total collaboration revenues were down 34% year over year to $63.5 million, which beat the Zacks Consensus Estimate of $62.5 million.
For 2023, the company reported a loss of $1.64 per share, narrower than the Zacks Consensus Estimate of a loss of $1.78. The company had incurred a loss of $2.91 per share in 2022.
Pipeline Update
FATE is focused on the development and manufacture of universal, off-the-shelf cell products using its proprietary induced pluripotent stem cell (iPSC) product platform. Its immuno-oncology pipeline includes off-the-shelf, iPSC-derived natural killer cells and T-cell product candidates. These candidates are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies and to target tumor-associated antigens using chimeric antigen receptors.
Earlier this month, the company was awarded $7.9 million by the California Institute for Regenerative Medicine to support the clinical development of FT819 in patients with systemic lupus erythematosus (SLE). The phase I SLE study will evaluate the safety, pharmacokinetics and anti-B-cell activity of a single dose of FT819 administered following a standard three-day chemotherapy conditioning regimen. FATE is currently engaged in study start-up activities at multiple clinical sites in the United States. Initial clinical data from the SLE study of FT819 is expected in 2024.
The company is also currently evaluating FT819 in a separate phase I study for the treatment of relapsed/refractory B-cell malignancies.
With ONO Pharmaceutical, the company is co-developing FT825/ONO-8250, a multiplexed-engineered, iPSC-derived CAR T-cell product candidate targeting human epidermal growth factor receptor 2-expressing solid tumors. During the reported quarter, Fate received clearance from the FDA to conduct a phase I study of FT825/ONO-8250 in patients with advanced solid tumors. In January 2024, the company initiated enrolling patients in the planned phase I study of the candidate.
The early-stage study will evaluate the safety, pharmacokinetics and activity of a single dose of FT825/ONO-8250 as a monotherapy and in combination with monoclonal antibody therapy in patients with advanced solid tumors.
Also, during the fourth quarter, Fate reported treating the first patient with its ADR-armed, CD19-targeted CAR NK cell product candidate, FT522, in a phase I study. Per the company, the early-stage study is evaluating the safety, pharmacokinetics and activity with and without administration of a standard three-day preconditioning regimen of FT522 in combination with Rituxan (rituximab) in patients with relapsed/refractory B-cell lymphoma.
Fate Therapeutics expects to share clinical data from the FT522 program in the second half of 2024.
Fate Therapeutics, Inc. Price, Consensus and EPS Surprise
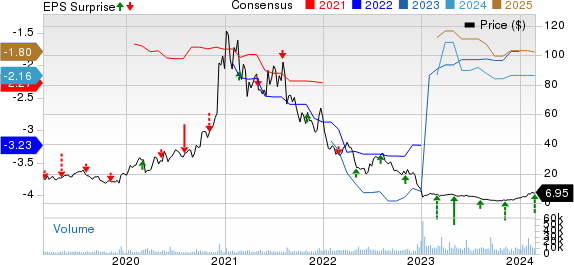
Fate Therapeutics, Inc. price-consensus-eps-surprise-chart | Fate Therapeutics, Inc. Quote
Zacks Rank and Stocks to Consider
FATE currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are Puma Biotechnology, Inc. PBYI, ADMA Biologics ADMA and Adicet Bio, Inc. ACET. While PBYI sports a Zacks Rank #1 (Strong Buy), ADMA & ACET carry a Zacks Rank #2 (Buy) each at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has remained constant at 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 69 cents to 71 cents. Over the past year, shares of PBYI have risen 45.1%.
PBYI's earnings beat estimates in three of the last four quarters and missed the same in one, delivering an average surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has remained constant at 2 cents. The consensus estimate for ADMA Biologics’ 2024 EPS is pegged at 22 cents. Over the past year, shares of ADMA have jumped 50%.
ADMA's earnings beat estimates in three of the trailing four quarters and met in one, delivering an average surprise of 63.57%.
In the past 30 days, the Zacks Consensus Estimate for Adicet Bio’s 2023 loss per share has remained constant at $3.39. During the same period, the consensus estimate for Adicet’s 2024 loss per share has narrowed from $2.29 to $1.81. In the past year, shares of ACET have plunged 69.9%.
ACET’s earnings beat estimates in two of the trailing four quarters and missed in the other two, delivering an average negative surprise of 8.36%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Adicet Bio, Inc. (ACET) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Fate Therapeutics, Inc. (FATE) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
