FibroGen (FGEN) Fails to Meet Goals in DMD Study, Stock Down 23%
FibroGen, Inc. FGEN announced the failure of its late-stage study of pamrevlumab for the treatment of ambulatory patients with Duchenne muscular dystrophy (DMD) on background systemic corticosteroids.
The phase III LELANTOS-2 study did not meet its primary endpoint of meaningful change in the North Star Ambulatory Assessment total score from baseline to week 52. Several secondary endpoints measured by change, including 4-stair climb velocity and 10-meter walk/run test, from baseline at week 52 did not meet in the study.
The stock of the company fell 22.7% during the after-market hours on Aug 29, 2023, in response to the study failure news.
Pamrevlumab was generally safe and overall well-tolerated in the study with mid-moderate treatment-emergent adverse events.
Pamrevlumab is FibroGen’s potential first-in-class connective tissue growth factor-inhibitor antibody. The global phase III LELANTOS-2 study enrolled 73 boys with ambulatory DMD, aged six to 12 years. It comprises two arms, receiving either a 35 mg/kg intravenous dose of pamrevlumab every two weeks or placebo through week 52.
Currently, FibroGen is engaged in evaluating the entire data from the LELANTOS-2 study, including other pre-specified endpoints, to determine the next steps for the program. The full results of the study will be presented at an upcoming medical conference.
We would like to remind the investors that in June 2023, FGEN announced the failure of the phase III LELANTOS-1 placebo-controlled study of pamrevlumab to treat non-ambulatory patients with DMD. The LELANTOS-1 study had failed to meet the primary endpoint of the Performance of the Upper Limb 2.0 score at week 52 compared with baseline.
Also in June, FGEN announced the failure of its late-stage study ZEPHYRUS-1 evaluating the safety and efficacy of pipeline candidate pamrevlumab in patients with idiopathic pulmonary fibrosis (IPF), following which the company decided to discontinue the second phase III ZEPHYRUS-2 study of pamrevlumab in patients with IPF.
Such back-to-back pipeline setbacks have been detrimental to the company’s stock. Year to date, shares of FibroGen have nosedived 92% compared with the industry’s 3.8% fall.
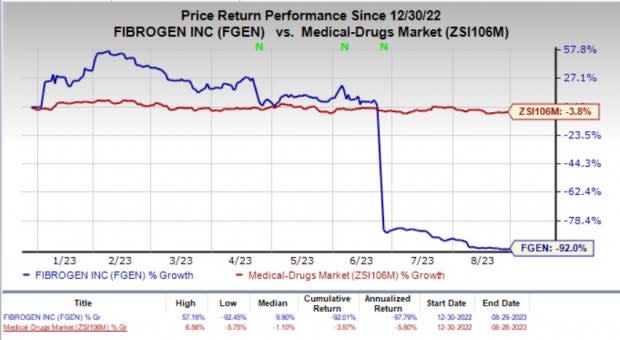
Image Source: Zacks Investment Research
Per FibroGen, DMD is a rare fatal neurodegenerative disease, affecting approximately one in every 5,000 newborn boys. It is characterized by muscle weakness, muscle loss, fibrosis and inflammation. DMD seriously impairs the quality of life as patients are often wheelchair-bound before the age of 12.
The company is also evaluating pamrevlumab for treating locally advanced pancreatic cancer in a phase III LAPIS study, expecting its top-line data in the first quarter of 2024. It is also being evaluated for metastatic pancreatic cancer in a phase II/III Precision Promise study, anticipating top-line results in the first half of 2024.
Currently, Sarepta Therapeutics SRPT is a market leader in the treatment of DMD. Sarepta’s commercial portfolio comprises four drugs, such as Exondys 51(eteplirsen), Vyondys 53 (golodirsen), Amondys 45 (casimersen) and Elevidys (SRP-9001), that have received the FDA’s accelerated approval.
The recently approved Elevidys is the first-ever gene therapy for DMD, which is indicated to treat ambulatory pediatric patients aged four through five years with a confirmed mutation in the DMD gene. Sarepta is currently conducting the phase III EMBARK confirmatory study seeking full approval for Elevidys in DMD indication.
Furthermore, the FDA has also agreed to a non-age-restricted expansion to Elevidys’ label, which is subject to the success of the EMBARK study. Sarepta anticipates its top-line results from the EMBARK study in the fourth quarter of 2023.
FibroGen, Inc Price and Consensus
FibroGen, Inc price-consensus-chart | FibroGen, Inc Quote
Zacks Rank & Other Stocks to Consider
FibroGen currently has a Zacks Rank #2 (Buy).
A couple of other top-ranked stocks in the pharma/biotech sector worth mentioning are J&J JNJ and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for J&J’s 2023 earnings per share has increased from $10.73 to $10.75. During the same period, the estimate for JNJ’s 2024 earnings per share has increased from $11.28 to $11.30. Year to date, shares of JNJ have lost 7%.
JNJ beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 5.58%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has gone up from 62 cents to 78 cents. The estimate for Corcept’s 2024 earnings per share has also improved from 61 cents to 83 cents. Year to date, shares of CORT have climbed 61.6%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
FibroGen, Inc (FGEN) : Free Stock Analysis Report
