FibroGen (FGEN) Plunges on Failure of Late-Stage IPF Study
Shares of FibroGen, Inc. FGEN were down 83.12% after the company faced another setback. The late-stage study ZEPHYRUS-1 evaluating the safety and efficacy of pipeline candidate pamrevlumab in patients with idiopathic pulmonary fibrosis (IPF) was unsuccessful.
The randomized, double-blind, placebo-controlled, multi-center phase III trial enrolled 356 patients with IPF. Patients were randomized (1:1) to receive either pamrevlumab or placebo for 48 weeks.
The study did not meet the primary endpoint of change from baseline in forced vital capacity (FVC) at week 48, as the mean decline in FVC from baseline to week 48 was 260 ml in the pamrevlumab arm compared with 330 ml in the placebo arm. The secondary endpoint of time to disease progression was also not met.
The candidate was generally safe and well tolerated, with the majority of treatment-emergent adverse events being mild or moderate.
As a result of disappointing results from the ZEPHYRUS-1 study, the company will now discontinue the second phase III study, ZEPHYRUS-2.
Earlier in the month, FGEN announced that the phase III LELANTOS-1 placebo-controlled trial of pamrevlumab for the treatment of non-ambulatory patients with Duchenne Muscular Dystrophy (DMD) on background corticosteroids did not meet the primary endpoint of the Performance of the Upper Limb 2.0 (PUL 2.0) score at week 52 compared with baseline.
The back-to-back pipeline setbacks weigh on shares. Shares of the company have plunged 84.6% in the year so far compared with the industry’s 3.5% decline.
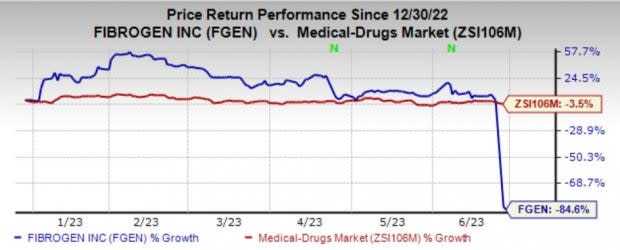
Image Source: Zacks Investment Research
FibroGen is also evaluating pamrevlumab for treating locally advanced pancreatic cancer (LAPIS) and data is expected in the first half of 2024. It is also being evaluated for metastatic pancreatic cancer (Precision Promise).
FibroGen plans to undertake a restructuring plan in the United States with the intent of extending its cash runway into 2026.
In May 2023, FibroGen announced that the late-stage MATTERHORN study of roxadustat for the treatment of anemia in patients with transfusion-dependent lower-risk myelodysplastic syndromes (MDS) did not meet its primary efficacy endpoint.
Roxadustat is currently approved in China, Europe, Japan and numerous other countries for treating anemia in chronic kidney disease (“CKD”) in non-dialysis-dependent and dialysis-dependent adult patients.
Zacks Rank and Stocks to Consider
FibroGen currently carries a Zacks Rank #3 (Hold). Some top-ranked stocks in the healthcare sector are Ligand Pharmaceuticals LGND and Novartis NVS.
LGND currently sports a Zacks Rank #1 (Strong Buy) and NVS carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Over the past 30 days, earnings estimates for LGND have increased by $1.09 to $5.25. LGND topped earnings estimates in two of the last four quarters and missed in the remaining two, the average surprise being 21.50%.
Over the past 60 days, NVS’ earnings estimates have increased to $6.74 from $6.60 for 2023. Novartis surpassed estimates in all the trailing four quarters, the average surprise being 5.15%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
FibroGen, Inc (FGEN) : Free Stock Analysis Report
