Fulcrum (FULC) Up as FDA Lifts Clinical Hold on SCD Candidate
Fulcrum Therapeutics FULC soared more than 55% this week, on Aug 22, as the company announced that the FDA has lifted the clinical hold that it had earlier placed on the investigational new drug (IND) application for FTX-6058, a potential treatment for sickle cell disease (SCD).
In February 2023, the FDA placed a full clinical hold on the IND application for FTX-6058 based on concerns raised by the agency on preclinical data that were submitted earlier in April, October and December of 2022. The FDA noted that these data showed hematological malignancies which are associated with other inhibitors of polycomb repressive complex 2.
With the FDA placing clinical hold, FULC halted enrollment and dosing in the phase Ib study of FTX-6058. Fulcrum also withdrew a separate IND application that it had previously submitted for FTX-6058 to treat beta thalassemia.
Fulcrum’s shares have lost 16.7% year to date compared with the industry's 13.1% decline.
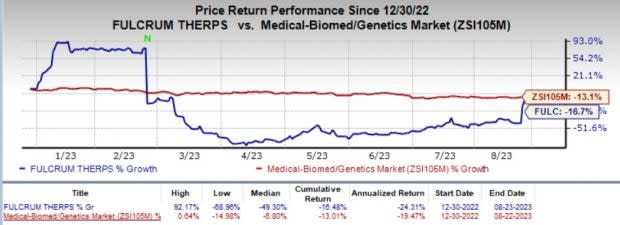
Image Source: Zacks Investment Research
Before the clinical hold was imposed, FTX-6058 was being evaluated in a phase Ib study for SCD. The study completed enrollment in the 6mg and 2mg dose cohorts. Interim data from the 6mg cohort showed an impressive 9.5% increase in fetal hemoglobin levels from baseline.
The FDA granted a Fast Track designation to FTX-6058 for treatment of patients with SCD in December 2022. The designation provides the company with additional market exclusivity and expedited regulatory paths.
SCD is a genetic disorder that affects red blood cells due to a mutation in the HBB gene. The disease has a significant unmet medical need, and if successfully developed and commercialized, FTX-6058has the potential to boost FULC’s prospects.
Fulcrum Therapeutics, Inc. Price and Consensus
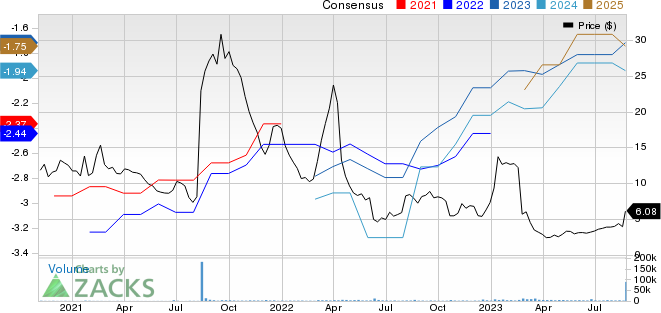
Fulcrum Therapeutics, Inc. price-consensus-chart | Fulcrum Therapeutics, Inc. Quote
Zacks Rank & Stocks to Consider
Fulcrum currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the same industry are ANI Pharmaceuticals ANIP, Annovis Bio ANVS and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate forANI Pharmaceuticals has gone up from $3.31 per share to $3.73 for 2023. The bottom-line estimate has gone up from $4.32 to $4.35 for 2024 during the same time frame. Shares of the company have rallied 58.6% year to date.
ANIP’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise 91.56%.
In the past 90 days, the Zacks Consensus Estimate for Annovis Bio has narrowed from a loss of $4.89 per share to a loss of $4.38 for 2023. The bottom-line estimate has narrowed from a loss of $3.18 to $2.77 for 2024 during the same time frame. Shares of the company have lost 3.2% year to date.
ANVS’ earnings beat estimates in three of the trailing four quarters and missed the mark in one, delivering an average surprise of 13.40%.
In the past 90 days, the Zacks Consensus Estimate for Corcept’s earnings has gone up from 62 cents per share to 78 cents for 2023. The bottom-line estimate has also improved from 61 cents to 83 cents for 2024 during the same time frame. Shares of the company have rallied 56.6% year to date.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
ANI Pharmaceuticals, Inc. (ANIP) : Free Stock Analysis Report
Fulcrum Therapeutics, Inc. (FULC) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report
