Fusion (FUSN) to Begin Clinical Study on Targeted Alpha Therapy
Fusion Pharmaceuticals FUSN, a clinical-stage oncology company, announced the clearance of an Investigational New Drug (IND) application by the FDA for two new radiopharmaceuticals, namely FPI-2068 and corresponding imaging analog FPI-2107. Shares were up almost 4.3% after trading hours on Apr 12 following the announcement. Fusion is jointly developing FPI-2068 with AstraZeneca AZN under the companies' multi-asset collaboration agreement.
FPI-2068 is a bio specific targeted alpha therapy (TAT) that aims to deliver radiation to several solid tumors that express EGFR and cMET. With the FDA clearance for the IND, Fusion Pharmaceuticals will soon begin clinical studies on the TAT.
This is a significant milestone for FUSN as it advances the novel TAT created by combining radiopharmaceutical expertise, actinium supply and manufacturing infrastructure with AZN's bispecific antibody. Fusion believes FPI-2068 will be the first TAT for two validated targets to enter the clinic.
FPI-2068 will be evaluated in a phase I study, making it the first program to enter clinical development under the company's previously announced collaboration agreement with AstraZeneca.
Fusion's shares have plunged 30.3% in the past year against the industry's 18.1% decline.
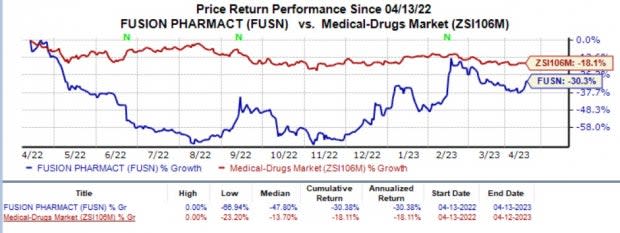
Image Source: Zacks Investment Research
The collaboration agreement in which FUSN will be operationally responsible for the phase I study, while AZN will be responsible for subsequent clinical development, was entered in 2020. The companies will share clinical development costs equally.
The collaboration included a joint discovery, development and the option to co-commercialize novel TATs leveraging Fusion's proprietary Fast-Clear linker technology platform with antibodies from AstraZeneca's oncology portfolio, as well as exploration of potential combination strategies involving existing assets in their respective portfolios.
Fusion has multiple early-stage development pipelines that use its TAT technology to target numerous types of cancer cells. In addition to a robust proprietary pipeline, the company has also collaborated with Merck MRK to evaluate FPI-1434 in combination with Merck's Keytruda (pembrolizumab) in patients with solid tumors expressing IGF-1R. The collaboration with Merck was entered in May 2021.
Fusion Pharmaceuticals Inc. Price and Consensus
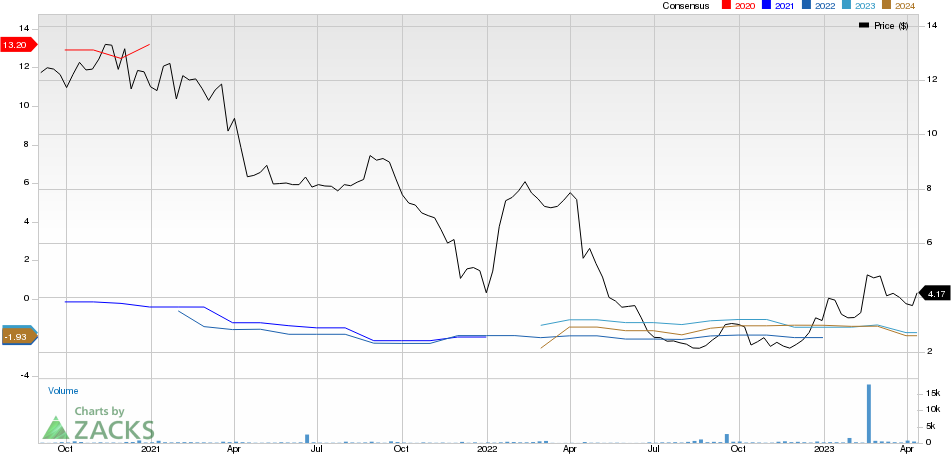
Fusion Pharmaceuticals Inc. price-consensus-chart | Fusion Pharmaceuticals Inc. Quote
Zacks Rank & Stocks to Consider
Fusion has a Zacks Rank #3 (Hold) currently.
A better-ranked stock for investors interested in the same sector is Allogene Therapeutics ALLO which carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today's Zacks #1 Rank (Strong Buy) stocks here.
Loss per share estimates for Allogene Therapeutics has narrowed from $2.83 to $2.44 for 2023 and from $2.69 to $2.46 for 2024 in the past 60 days.
The company's earnings beat estimates in each of the trailing four quarters, the average surprise being 8.33%. ALLO's shares have plunged 47.6% in the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Fusion Pharmaceuticals Inc. (FUSN) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report
