Germany In-Vitro Diagnostics Market Report 2023: Sector to Reach $5.11 Billion by 2028 at a 2.5% CAGR


German In-Vitro Diagnostics Market

Dublin, June 08, 2023 (GLOBE NEWSWIRE) -- The "Germany In-Vitro Diagnostics Market, Size, Forecast 2023-2028, Industry Trends, Growth, Share, Outlook, Impact of Inflation, Opportunity Company Analysis" report has been added to ResearchAndMarkets.com's offering.
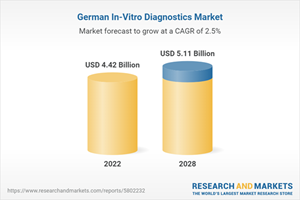
Germany In-Vitro Diagnostics Market will reach US$ 5.11 Billion in 2028, according to the publisher. IVD tests have several uses, including diagnosis for infectious diseases, cancer, autoimmune diseases, and genetic disorders.
They also play a crucial role in screening and early disease detection, disease monitoring and management, risk assessment and personalized medicine, and transplantation compatibility. In addition, IVD tests are also widely used in pregnancy, prenatal care, and forensic and drug testing.
The in-vitro diagnostics market in Germany is growing because of the rising number of chronic illnesses, a growing aging population, rising demand for accurate diagnosis, and favorable government initiatives.
For instance, Germany witnessed approximately 628,519 new cancer cases, as GLOBOCAN reported. Among females, breast and colorectal cancer were more prevalent, while among males, prostate and lung cancer were more common in the country.
Germany In-Vitro Diagnostics Market shall expand at a CAGR of 2.44% from 2022 to 2028
Germany IVD market is one of the Europe's largest, driven by its robust healthcare system and population of over 83 million people. With advanced healthcare infrastructure, including hospitals, labs, and research institutions, Germany fosters technological innovation and leads in developing cutting-edge diagnostics technologies. The market spans various applications, such as infectious diseases, cancer diagnostics, and genetic testing, reflecting Germany's focus on preventive medicine and personalized healthcare.
Adhering to EU regulations, Germany ensures safety and performance standards for IVD products. In addition, the country's comprehensive healthcare system, with a reimbursement mechanism, promotes accessibility and financial viability for patients. Multinational corporations and local manufacturers enhance the market's competitiveness with innovative products and solutions. Hence, the market value for Germany's in-vitro Diagnostics Market was US$ 4.42 Billion in 2022.
Services in the Germany In-vitro Diagnostics Market shall experience thriving growth
The product segments in Germany in-vitro diagnostics market include Services, Instruments, and Reagents. Increasing demand for outsourced services allows healthcare facilities to optimize operations and focus on core competencies, enhancing efficiency and cost reduction. Technological advancements like automation and data analytics enable service providers to offer more efficient and accurate diagnostic services. The complexity of diagnostic testing, including molecular diagnostics and genetic testing, has led to a greater reliance on specialized service providers.
Outsourcing diagnostic services also brings cost-effectiveness and resource optimization, avoiding heavy upfront investments and allowing the allocation of resources to critical patient care areas. Specialized service providers excel in quality assurance and compliance with regulatory requirements, ensuring accurate and reliable results. Collaboration and integration with healthcare systems enable seamless coordination of diagnostic services, facilitating effective patient care.
Rapid tests are gaining fame in Germany In-vitro Diagnostics Market
Germany in-vitro diagnostics market offers a variety of test types, such as ELISA & CLIA, PCR, Rapid Test, Fluorescence Immunoassays (FIA), In Situ Hybridization, Transcription Mediated Amplification, Sequencing, Colorimetric Immunoassay, Radioimmunoassay (RIA), Isothermal Nucleic Acid Amplification Technology, and others. Rapid tests have become increasingly popular due to their quick and efficient results, eliminating the need for lengthy laboratory processing times. They enable healthcare professionals to perform diagnostics tests at the point of care and play a crucial role in various screening programs.
Rapid tests have gained prominence with the growing emphasis on early detection and prevention, especially in outpatient settings. Technology advancements have enhanced their performance and accuracy, expanding the range of conditions and pathogens detected. Rapid tests are also crucial tools in public health emergency preparedness and are often designed for ease of use, requiring minimal training. The COVID-19 pandemic has significantly boosted their demand worldwide, helping to control the spread of the virus.
Cardiology application is the fastest growing in Germany In-vitro Diagnostics Market
In the German in-vitro Diagnostics Market, several applications hold great importance, encompassing Infectious Diseases, Diabetes, Cardiology, Oncology, Nephrology, Autoimmune Diseases, Drug Testing, and various other applications. Cardiovascular diseases drive demand for advanced cardiology diagnostics in Germany. In-vitro diagnostics play a crucial role in early detection and management. Technological advancements improve accuracy, efficiency, and risk assessment.
In addition, the aging population increases the demand for cardiac diagnostics. In-vitro tests provide valuable information on biomarkers and genetic markers. Preventive healthcare emphasizes early detection and intervention, with non-invasive diagnostics helping prevent cardiac diseases. Point-of-care testing expands access to immediate diagnostics. Favorable reimbursement policies drive adoption and market growth.
Immunoassay dominates Germany In-vitro Diagnostics Market due to its versatile applications, automation capabilities, and extensive assay catalog
In Germany In-vitro diagnostics market, the technologies employed include Immunoassay, Clinical Chemistry, Molecular Diagnostics/Genetics, Hematology, Microbiology, Coagulation, and other related technologies. Immunoassay techniques have many applications across medical disciplines like infectious diseases, oncology, endocrinology, autoimmune diseases, and allergy testing. They are famous for their high sensitivity and specificity, enabling accurate detection and quantification of target analytes.
Immunoassay platforms offer automation and increased throughput capabilities, making them efficient for analyzing large samples. The extensive catalog of validated assays, regulatory approval, and ongoing research contributes to their reliability and performance. In addition, immunoassays are cost-effective and benefit from Germany's well-developed healthcare infrastructure and skilled workforce in laboratory medicine, further drive their dominance in the market.
Hospitals maintain their dominant position in the Germany in-vitro diagnostics market
Germany's in-vitro diagnostics market classifies end-users into four categories: Hospitals, Laboratories, Home-Care, and Others. Hospitals dominate Germany's in-vitro diagnostics market due to their comprehensive healthcare services, advanced facilities and equipment, availability of specialized departments, collaboration with healthcare professionals, accessibility and convenience, referral system, quality assurance and accreditation, and affiliation with research and academic institutions. These factors collectively establish hospitals as the primary location for conducting a wide range of in-vitro diagnostic tests, attracting patients and healthcare providers.
Products - Market breakup from 3 Viewpoints:
1. Services
2. Instruments
3. Reagents
Test Types - Market breakup from 11 Viewpoints:
1. ELISA & CLIA
2. PCR
3. Rapid Test
4. Fluorescence Immunoassays (FIA)
5. In Situ Hybridization
6. Transcription Mediated Amplification
7. Sequencing
8. Colorimetric Immunoassay
9. Radioimmunoassay (RIA)
10. Isothermal Nucleic Acid Amplification Technology
11. Others
Application - Market breakup from 8 Viewpoints:
1. Infectious Diseases
2. Diabetes
3. Cardiology
4. Oncology
5. Nephrology
6. Autoimmune Diseases
7. Drug Testing
8. Others
Technology - Market breakup from 7 Viewpoints:
1. Immunoassay
2. Clinical Chemistry
3. Molecular Diagnostics/Genetics
4. Hematology
5. Microbiology
6. Coagulation
7. Others
End-Users - Market breakup from 4 Viewpoints:
1. Hospitals
2. Laboratories
3. Home - Care
4. Others
Company has been covered from 3 Viewpoints:
Overview
Recent Development
Revenue
Key Attributes:
Report Attribute | Details |
No. of Pages | 180 |
Forecast Period | 2022 - 2028 |
Estimated Market Value (USD) in 2022 | $4.42 Billion |
Forecasted Market Value (USD) by 2028 | $5.11 Billion |
Compound Annual Growth Rate | 2.4% |
Regions Covered | Germany |
Key Topics Covered:
1. Introduction
2. Research & Methodology
3. Executive Summary
4. Market Dynamics
5. Porter's Five Forces
6. SWOT Analysis
7. Germany In-Vitro Diagnostics (IVD) Market
8. Market Share - Germany In-Vitro Diagnostics (IVD)
9. Test Types - Germany In-Vitro Diagnostics (IVD) Market
9.1 ELISA & CLIA
9.2 PCR
9.3 Rapid Test
9.4 Fluorescence Immunoassays (FIA)
9.5 In Situ Hybridization
9.6 Transcription Mediated Amplification
9.7 Sequencing
9.8 Colorimetric Immunoassay
9.9 Radioimmunoassay (RIA)
9.10 Isothermal Nucleic Acid Amplification Technology
9.11 Others
10. Product Types - Germany In-Vitro Diagnostics (IVD) Market
10.1 Services
10.2 Instruments
10.3 Reagents
11. Technology - Germany In-Vitro Diagnostics (IVD) Market
11.1 Immunoassay
11.2 Clinical Chemistry
11.3 Molecular Diagnostics/Genetics
11.4 Hematology
11.5 Microbiology
11.6 Coagulation
11.7 Others
12. Application - Germany In-Vitro Diagnostics (IVD) Market
12.1 Infectious Disease
12.2 Diabetes
12.3 Cardiology
12.4 Oncology
12.5 Nephrology
12.6 Autoimmune Diseases
12.7 Drug Testing
12.8 Other Applications
13. End User - Germany In-Vitro Diagnostics (IVD) Market
13.1 Hospitals
13.2 Laboratories
13.3 Home-Care
13.4 Others
14. Government Rules & Regulation
15. Reimbursement
15.1 Public
15.2 Private& Insurance
16. Company Analysis
Companies Mentioned
Roche Diagnostics
Abbott Diagnostics
Siemens Healthineers
Danaher Corporation
Thermo Fisher Scientific
Sysmex Corporation
For more information about this report visit https://www.researchandmarkets.com/r/z5gvfm
About ResearchAndMarkets.com
ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
Attachment
CONTACT: CONTACT: ResearchAndMarkets.com Laura Wood,Senior Press Manager press@researchandmarkets.com For E.S.T Office Hours Call 1-917-300-0470 For U.S./ CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900

