Gilead's (GILD) Veklury Gets CHMP Nod in Damaged Liver Patients
Gilead Sciences, Inc. GILD announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has given a positive opinion for the use of Veklury (remdesivir) to treat COVID-19 in people with mild to severe hepatic impairment in the European Union (EU). Veklury is the company’s anti-viral drug.
Hepatic impairment is a condition in which the liver stops working properly due to damage from liver disease and cannot perform its basic functions.
The positive CHMP opinion is based on results from a phase I study in people with hepatic impairment (GS-US-540-9014), wherein no new safety signals were observed. Gilead conducted this multicenter, open-label, single-dose study to evaluate the pharmacokinetics and safety of Veklury and its metabolites in participants with normal hepatic function and moderate or severe hepatic impairment.
The CHMP recommendation, considering the GS-US-540-9014 study results, for the use of Veklury in people with hepatic impairment requires no dose adjustment or liver function testing before or during treatment with the drug.
Although not binding in nature, the EMA will review the CHMP’s recommendation for marketing authorization to Veklury for the expanded indication in the EU. Subject to approval, Veklury can become the first and only authorized anti-viral COVID-19 treatment that can be used across all stages of liver disease in the EU.
Shares of Gilead have lost 11.7% year to date compared with the industry’s decline of 14.6%.
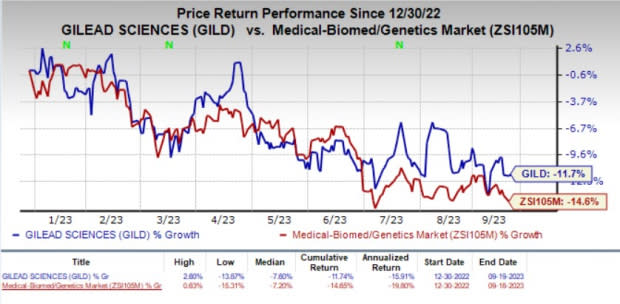
Image Source: Zacks Investment Research
Per Gilead, most liver disease patients in the world are from Europe and cases are expected to grow across many countries. People suffering from liver disease are highly susceptible to COVID-19 with an increased risk of morbidity and mortality. There are limited safe and effective options for the treatment of COVID-19 in people with severe hepatic impairment. This represents a significant unmet medical need.
Last month, the FDA approved the label expansion of Veklury in the same indication for which the drug received positive CHMP opinion in the EU. Veklury is now approved in the United States without any dose adjustments for the treatment of COVID-19 in patients with mild, moderate or severe hepatic impairment.
At the peak of the pandemic, Veklury was approved in the United States and EU for the treatment of COVID-19 in adults and pediatric patients who are hospitalized or have mild-to-moderate COVID-19 and are at high risk for progression to severe COVID-19, including hospitalization or death.
In July 2023, the FDA approved the label expansion of Veklury to include COVID-19 patients with severe renal impairment, including those on dialysis. The EMA has also approved the drug’s label expansion to treat COVID-19 in people with severe renal impairment, including those on dialysis, in June 2023, following the positive CHMP opinion in May 2023.
The outbreak of COVID-19 in 2020 and the need for urgent treatment for those infected significantly boosted Veklury sales in 2021. However, sales are on a declining trajectory, thereafter, with the easing of the pandemic.
Veklury sales plunged 58% year over year to $829 million in the first half of 2023, primarily due to lower rates of COVID-19-related hospitalizations.
Gilead Sciences, Inc. Price and Consensus
Gilead Sciences, Inc. price-consensus-chart | Gilead Sciences, Inc. Quote
Zacks Rank and Stocks to Consider
Gilead currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall medical sector, worth mentioning, are Dynavax Technologies DVAX, Corcept Therapeutics CORT and Better Therapeutics BTTX, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Dynavax’s 2023 loss per share has narrowed from 24 cents to 23 cents. The estimate for Dynavax’s 2024 earnings per share is currently pegged at 3 cents. Year to date, shares of DVAX have risen by 29%.
DVAX’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 25.78%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has remained constant at 78 cents. The estimate for Corcept’s 2024 earnings per share has also remained constant at 83 cents. Year to date, shares of CORT have climbed 62.3%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
In the past 30 days, the Zacks Consensus Estimate for Better Therapeutics’ 2023 loss per share has remained constant at 98 cents. During the same period, Better Therapeutics’ 2024 loss per share has also remained constant at 80 cents. Year to date, shares of BTTX have lost 59.3%.
BTTX’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 24.22%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Better Therapeutics, Inc. (BTTX) : Free Stock Analysis Report
