Glaxo (GSK), Vir Intramuscular Sotrovimab Meets Study Endpoint
GlaxoSmithKline GSK and partner Vir Biotech VIR announced positive top-line data from a phase III study — COMET-TAIL — evaluating the intramuscular administration of their COVID-19 therapy candidate, sotrovimab. Data from the study demonstrated that the intramuscular administration of the candidate met the primary endpoint of non-inferiority to intravenous administration in reducing hospitalization and risk of death in adults with mild-to-moderate COVID-19 who are at high risk of progression to severe disease.
We note that Glaxo and Vir’s sotrovimab, as an intravenous administration, was granted emergency use authorization (“EUA”) for treating mild-to-moderate COVID-19 in adult and pediatric patients in May. The intravenous drug is available with the tradename of Xevudy. The same was rendered a positive scientific opinion for the early treatment of COVID-19 in a similar patient population in Europe by the Committee for Human Medicinal Products (CHMP) in May. The investigational therapy is also authorized for temporary/conditional use in several other countries including Japan and Canada.
Data from the COMET-TAIL study showed that both intramuscular and intravenous administration of sotrovimab offered similar efficacy for high-risk populations. While the rate of progression to hospitalization for more than 24 hours or death was 2.7% for the intramuscular administration, the rate was 1.3% for the intravenous administration, with an adjusted difference of 1.07%. The non-inferiority margin of the intramuscular administration was within the predetermined 3.5% mark, the study’s primary endpoint set after consultation with the FDA.
Based on the non-inferiority of the intramuscular administration of sotrovimab, Glaxo and Vir are planning for regulatory submissions of the same globally. The companies are also in discussion with the FDA to include the intramuscular administration in the existing EUA for sotrovimab.
So far this year, Glaxo’s shares have risen 17.8% compared with the industry’s 16.2% increase.
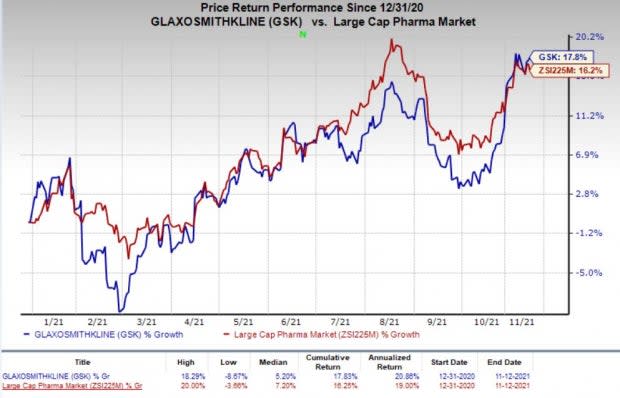
Image Source: Zacks Investment Research
A potential EUA for the intramuscular administration of sotrovimab will provide a more convenient administration option for COVID-19 patients compared to the intravenous administration. We note that there are two other FDA-authorized treatment monoclonal antibodies available for non-hospitalized COVID-19 patients — Eli Lilly’s LLY cocktail therapy, bamlanivimab plus etesevimab and Regeneron’s REGN antibody cocktail, REGEN-COV (casirivimab and imdevimab).
Lilly’s COVID-19 antibody cocktail, bamlanivimab plus etesevimab, was granted emergency approval by the FDA in February 2021 to treat mild-to-moderate COVID-19 in high-risk patients based on data from the BLAZE-1 study.
In September, the FDA expanded the EUA for the cocktail antibody medicine to include the post-exposure prevention (prophylaxis) for COVID-19 indication. Lilly’s cocktail medicine generated revenues of $217.1 million in the third quarter of 2021.
Regeneron’s antibody cocktail, REGEN-COV comprises two monoclonal antibodies, casirivimab and imdevimab. It has become a significant contributor to Regeneron’s top line in the recent quarters.
GlaxoSmithKline plc Price
GlaxoSmithKline plc price | GlaxoSmithKline plc Quote
Zacks Rank
Glaxo currently has a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
