Here's Why Rocket Pharmaceuticals (RCKT) Rises 53.8% in a Week
Shares of Rocket Pharmaceuticals, Inc. RCKT have skyrocketed 53.8% in the past week against the industry’s decline of 0.4%.
The surge is mostly attributable to the late-stage biotechnology company’s positive update on the phase II trial for investigational gene therapy RP-A501 for the treatment of Danon Disease.
Earlier in the week, Rocket Pharmaceuticals announced that it reached an alignment with the FDA on the mid-stage study design for RP-A501 in Danon Disease. Investors cheered the news.
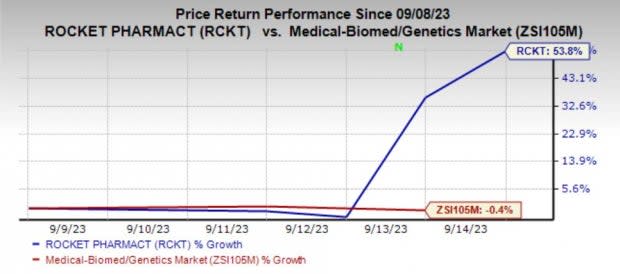
Image Source: Zacks Investment Research
Danon Disease, a uniformly fatal inherited cardiomyopathy, is caused by mutations in the lysosome-associated membrane protein 2 (LAMP-2) gene.
The global, single-arm, multi-center phase II study will evaluate the efficacy and safety of RP-A501 in 12 patients with Danon Disease, including a pediatric safety run-in (n=2), with a natural history comparator and a dose level of 6.7 x 1013 GC/kg.
The study will assess the efficacy of RP-A501 as measured by the biomarker-based co-primary endpoint consisting of improvements in LAMP-2 protein expression (≥ Grade 1, as measured by immunohistochemistry) and reductions in left ventricular mass to support accelerated approval.
While the key secondary endpoint is a change in troponin, additional secondary endpoints will include natriuretic peptides, the Kansas City Cardiomyopathy Questionnaire, the New York Heart Association class, event-free survival to 24 months and treatment-emergent safety events. The company expects these endpoints could support full approval with longer-term follow-up.
A global natural history study will serve as an external comparator and run concurrently with this study.
In order to enable the initiation of EU study activities, the filing of the Clinical Trial Application /Investigational Medicinal Product Dossier for RP-A501 is expected in the third quarter. Rocket has secured an ICD-10 code from CMS for LAMP2 deficiency in Danon Disease.
The successful development of this cardiac gene therapy for this rare inherited disorder will be a significant boost for the company.
Concurrently, the company announced that it is offering 7.8 million shares of its common stock and, to certain investors, pre-funded warrants to purchase 3.12 million shares of common stock. The company expects to receive proceeds of $175 million from the offering.
Apart from RP-A501, the company is also evaluating RP-A601 (PKP2 Arrhythmogenic Cardiomyopathy), RP-L201 (Leukocyte Adhesion Deficiency-I), RP-L102 (Fanconi Anemia) and RP-L301 (Pyruvate Kinase Deficiency).
Zacks Rank and Stocks to Consider
Rocket Pharmaceuticals currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the overall healthcare sector are Dynavax Technologies DVAX and Exelixis EXEL, currently carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Loss estimates for Dynavax for 2023 have narrowed to 24 cents from 56 cents in the past 90 days, while earnings estimates for 2024 are pegged at 2 cents per share.
Shares of EXEL have gained 38.5% year to date. Earnings estimates for 2023 have risen by 9 cents to 98 cents.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
Rocket Pharmaceuticals, Inc. (RCKT) : Free Stock Analysis Report
