Heron (HRTX) Gains 19% on FDA Nod for Zynrelef Label Expansion
Heron Therapeutics, Inc. HRTX announced that the FDA has approved a supplemental new drug application (sNDA), seeking label expansion of Zynrelef (bupivacaine and meloxicam) extended-release solution for the second time since the initial approval.
Zynrelef is the company’s dual-acting local anesthetic that delivers a fixed-dose combination of the local anesthetic bupivacaine and a low dose of the nonsteroidal anti-inflammatory drug meloxicam.
Zynrelef is presently approved for foot and ankle, small-to-medium open abdominal, and lower extremity total joint arthroplasty surgical procedures in adults.
The latest approval will expand Zynrelef’s indication for soft tissue and orthopedic surgical procedures, including foot and ankle and other procedures in which direct exposure to articular cartilage is avoided.
It marks a significant development, broadening its application to encompass an estimated 13 million medical procedures annually. This reflects an impressive 86% surge compared with previously indicated procedures.
Heron’s stock surged 18.9% on Jan 24, in response to the encouraging news. Over the past year, shares of HRTX have gained 0.6% against the industry’s 6.1% decline.
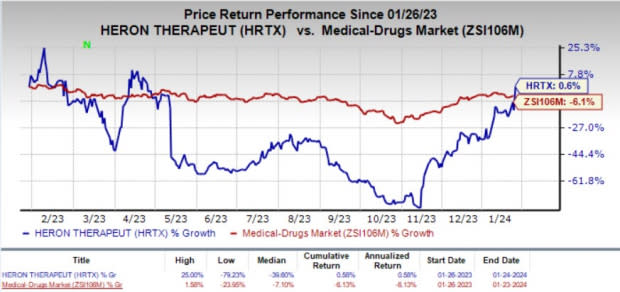
Image Source: Zacks Investment Research
The inclusion of these indications demonstrates the effectiveness of Zynrelef in addressing a wide range of medical needs in pain management.
These studies revealed no distinctive safety concerns following Zynrelef administration. The bupivacaine and meloxicam blood concentrations in patients were also consistent with previously available post-administration data. Such observations contribute to the overall confidence in the drug's reliability and suitability for expanded usage in diverse medical procedures.
The drug was initially approved around mid-2021 as thefirst and only extended-release solution for use in adults for soft tissue or periarticular instillation to produce postsurgical analgesia for up to 72 hours after bunionectomy, open inguinal herniorrhaphy and total knee arthroplasty.
The FDA approval was based on positive results from phase III studies of Zynrelef, demonstrating its superiority to bupivacaine solution, the current standard of care. Such parameters of superiority compared with bupivacaine included lower pain scores, opioid consumption and fewer patients experiencing severe pain.
Later the same year, Zynrelef’s label was expanded significantly to include use in adults for soft tissue or periarticular instillation to produce postsurgical analgesia for up to 72 hours after foot and ankle, small-to-medium open abdominal and lower extremity total joint arthroplasty surgical procedures.
Heron Therapeutics, Inc. Price and Consensus
Heron Therapeutics, Inc. price-consensus-chart | Heron Therapeutics, Inc. Quote
Zacks Rank and Other Stocks to Consider
Heron currently carries a Zacks Rank #2 (Buy).
Some other top-ranked stocks from the drug/biotech industry worth mentioning are Puma Biotechnology, Inc. PBYI, CytomX Therapeutics CTMX and Journey Medical DERM, each sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has increased from 72 cents to 73 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 64 cents to 69 cents. Over the past year, shares of PBYI have gained 13.1%.
PBYI beat estimates in three of the last four quarters while missing on one occasion, delivering a four-quarter average earnings surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for CytomX’s 2023 EPS has remained constant at 2 cents. The consensus estimate for CytomX’s 2024 loss per share is currently pegged at 6 cents. Over the past year, shares of CTMX have plunged 45%.
CTMX beat estimates in three of the trailing four quarters and missed the mark on one occasion, delivering an average earnings surprise of 45.44%.
In the past 30 days, the Zacks Consensus Estimate for Journey Medical’s 2023 loss per share has remained constant at 16 cents. During the same period, the consensus estimate for Journey Medical’s 2024 loss per share has remained constant at 69 cents. Over the past year, shares of DERM have skyrocketed 158%.
DERM beat estimates in one of the trailing four quarters and missed on the other three occasions, delivering an average earnings surprise of 118.25%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Heron Therapeutics, Inc. (HRTX) : Free Stock Analysis Report
Journey Medical Corporation (DERM) : Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX) : Free Stock Analysis Report
