Hikma exceeds 150 injectable medicines in the US with launch of Dobutamine Injection, USP
LONDON, June 15, 2023 /PRNewswire/ -- Hikma Pharmaceuticals PLC (Hikma), the multinational pharmaceutical company, has launched Dobutamine Injection, USP, in a 250mg/20mL vial in the US. With this launch, Hikma's growing US portfolio of sterile injectable medicines now exceeds 150 products spanning a wide range of therapeutic areas and dosage forms.
"Our portfolio of more than 150 injectable medicines has expanded by more than 50 percent over the last three years1, driven by customer needs, an expansion of our capabilities and product offerings, and the continued recognition of Hikma as a reliable, high-quality supplier of sterile injectable medicines," said Riad Mishlawi, President, Injectables, Hikma. "As a top-three supplier of generic injectable medicines by volume in the US2, we are proud of the important role we play in the US health care system. We are committed to further growing our portfolio and capabilities and continuing to supply essential medicines to US hospitals, doctors, and patients."
In addition to its growing US portfolio, Hikma now markets approximately 30 sterile injectable medicines in Canada, with plans to launch up to 13 additional products this year. The Company has also launched Hikma 503B, a new sterile compounding business focused on providing high-quality, ready-to-administer injectable medications that are customized to the specific needs of patients in the US.
About Dobutamine Injection, USP
Dobutamine Injection, USP has been launched in the US and is indicated when parenteral therapy is necessary for inotropic support in the short-term treatment of adults with cardiac decompensation due to depressed contractility resulting either from organic heart disease or from cardiac surgical procedures.
According to IQVIA, US sales of Dobutamine Injection, USP, in 250mg/20mL were approximately $2 million in the 12 months ending April 2023.
Enquiries | |
Hikma Pharmaceuticals PLC | |
Susan Ringdal | +44 (0)20 7399 2760/ +44 7776 477050 |
EVP, Strategic Planning and Global Affairs | |
Steve Weiss | +1 732 788 8279 |
David Belian | +1 848 254 4875 |
US Communications and Public Affairs |
About Hikma
(LSE: HIK) (NASDAQ Dubai: HIK) (OTC: HKMPY) (rated BBB-/stable S&P and BBB-/stable Fitch)
Hikma helps put better health within reach every day for millions of people around the world. For more than 40 years, we've been creating high-quality medicines and making them accessible to the people who need them. Headquartered in the UK, we are a global company with a local presence across the United States (US), the Middle East and North Africa (MENA) and Europe, and we use our unique insight and expertise to transform cutting-edge science into innovative solutions that transform people's lives. We're committed to our customers, and the people they care for, and by thinking creatively and acting practically, we provide them with a broad range of branded and non-branded generic medicines. Together, our 8,800 colleagues are helping to shape a healthier world that enriches all our communities. We are a leading licensing partner, and through our venture capital arm, are helping bring innovative health technologies to people around the world. For more information, please visit: www.hikma.com
This product has been approved for marketing in the United States by the US FDA. This product approval does not confer the right on Hikma, or any other party, to market this product outside the United States.
Important Safety Information for Dobutamine Injection, USP, in 250mg/20mL:
CONTRAINDICATIONS
Dobutamine is contraindicated in patients with idiopathic hypertrophic subaortic stenosis and in patients who have shown previous manifestations of hypersensitivity to dobutamine.
WARNINGS & PRECAUTIONS
Increase in Heart Rate or Blood Pressure: Dobutamine may cause a marked increase in heart rate or blood pressure, especially systolic pressure. Approximately 10% of adult patients in clinical studies have had rate increases of 30 beats/minute or more, and about 7.5% have had a 50 mm Hg or greater increase in systolic pressure. Usually, reduction of dosage promptly reverses these effects. Because dobutamine facilitates atrioventricular conduction, patients with atrial fibrillation are at risk of developing rapid ventricular response. In patients who have atrial fibrillation with rapid ventricular response, a digitalis preparation should be used prior to institution of therapy with dobutamine. Patients with preexisting hypertension appear to face an increased risk of developing an exaggerated pressor response.
Ectopic Activity: Dobutamine may precipitate or exacerbate ventricular ectopic activity, but it rarely has caused ventricular tachycardia.
Hypersensitivity: Reactions suggestive of hypersensitivity associated with administration of dobutamine including skin rash, fever, eosinophilia, and bronchospasm, have been reported occasionally.
Dobutamine contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes, in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
During the administration of dobutamine, as with any adrenergic agent, ECG and blood pressure should be continuously monitored. In addition, pulmonary wedge pressure and cardiac output should be monitored whenever possible to aid in the safe and effective infusion of dobutamine.
Hypovolemia should be corrected with suitable volume expanders before treatment with dobutamine is instituted.
No improvement may be observed in the presence of marked mechanical obstruction, such as severe valvular aortic stenosis.
Usage Following Acute Myocardial Infarction - Clinical experience with dobutamine following myocardial infarction has been insufficient to establish the safety of the drug for this use. There is concern that any agent that increases contractile force and heart rate may increase the size of an infarction by intensifying ischemia, but it is not known whether dobutamine does so.
Laboratory Tests - Dobutamine, like other ß2-agonists, can produce a mild reduction in serum potassium concentration, rarely to hypokalemic levels. Accordingly, consideration should be given to monitoring serum potassium.
ADVERSE REACTIONS
Increased Heart Rate, Blood Pressure, and Ventricular Ectopic Activity - A 10- to 20-mm increase in systolic blood pressure and an increase in heart rate of 5 to 15 beats/minute have been noted in most patients (see WARNINGS regarding exaggerated chronotropic and pressor effects). Approximately 5% of patients have had increased premature ventricular beats during infusions. These effects are dose related.
Hypotension - Precipitous decreases in blood pressure have occasionally been described in association with dobutamine therapy. Decreasing the dose or discontinuing the infusion typically results in rapid return of blood pressure to baseline values. In rare cases, however, intervention may be required and reversibility may not be immediate.
Reactions at Sites of Intravenous Infusion - Phlebitis has occasionally been reported. Local inflammatory changes have been described following inadvertent infiltration. Isolated cases of cutaneous necrosis (destruction of skin tissue) have been reported.
Miscellaneous Uncommon Effects - The following adverse effects have been reported in 1% to 3% of patients: nausea, headache, anginal pain, nonspecific chest pain, palpitations, and shortness of breath.
Isolated cases of thrombocytopenia have been reported.
Administration of dobutamine, like other catecholamines, can produce a mild reduction in serum potassium concentration, rarely to hypokalemic levels (see PRECAUTIONS).
DRUG INTERACTIONS
Animal studies indicate that dobutamine may be ineffective if the patient has recently received a ß-blocking drug. In such a case, the peripheral vascular resistance may increase.
Preliminary studies indicate that the concomitant use of dobutamine and nitroprusside results in a higher cardiac output and, usually, a lower pulmonary wedge pressure than when either drug is used alone.
There was no evidence of drug interactions in clinical studies in which dobutamine was administered concurrently with other drugs, including digitalis preparations, furosemide, spironolactone, lidocaine, glyceryl trinitrate, isosorbide dinitrate, morphine, atropine, heparin, protamine, potassium chloride, folic acid, and acetaminophen.
USE IN SPECIFIC POPULATIONS
Carcinogenesis, Mutagenesis, Impairment of Fertility - Studies to evaluate the carcinogenic or mutagenic potential of dobutamine, or its potential to affect fertility, have not been conducted.
Pregnancy–Teratogenic Effects - Reproduction studies performed in rats at doses up to the normal human dose (10 mcg/kg/min for 24 h, total daily dose of 14.4 mg/kg), and in rabbits at doses up to twice the normal human dose, have revealed no evidence of harm to the fetus due to dobutamine. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Labor and Delivery - The effect of dobutamine on labor and delivery is unknown.
Nursing Mothers - It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when dobutamine is administered to a nursing woman. If a mother requires dobutamine treatment, breastfeeding should be discontinued for the duration of the treatment.
Pediatric Use - Dobutamine has been shown to increase cardiac output and systemic pressure in pediatric patients of every age group. In premature neonates, however, dobutamine is less effective than dopamine in raising systemic blood pressure without causing undue tachycardia, and dobutamine has not been shown to provide any added benefit when given to such infants already receiving optimal infusions of dopamine.
Geriatric Use - Of the 1893 patients in clinical studies who were treated with dobutamine, 930 (49.1%) were 65 and older. No overall differences in safety or effectiveness were observed between these and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or drug therapy.
DOSAGE AND ADMINISTRATION
Note - Do not add dobutamine to 5% Sodium Bicarbonate Injection or to any other strongly alkaline solution. Because of potential physical incompatibilities, it is recommended that dobutamine not be mixed with other drugs in the same solution. Dobutamine should not be used in conjunction with other agents or diluents containing both sodium bisulfite and ethanol.
Preparation and Stability - At the time of administration, dobutamine must be further diluted in an IV container to at least a 50 mL solution using one of the following intravenous solutions as a diluent: 5% Dextrose Injection, 5% Dextrose and 0.45% Sodium Chloride Injection, 5% Dextrose and 0.9% Sodium Chloride Injection, 10% Dextrose Injection, Isolyte® M with 5% Dextrose Injection, Lactated Ringer's Injection, 5% Dextrose in Lactated Ringer's Injection, Normosol®-M in D5-W, 20% Osmitrol® in Water for Injection, 0.9% Sodium Chloride Injection, or Sodium Lactate Injection. Intravenous solutions should be used within 24 hours.
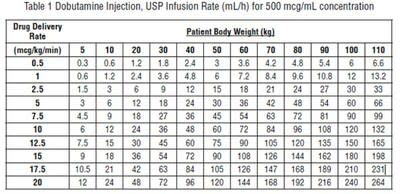
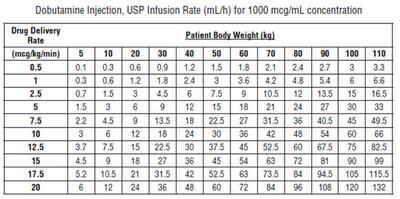
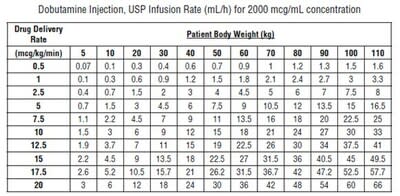
Recommended Dosage - Infusion of dobutamine should be started at a low rate (0.5 to1mcg/kg/min) and titrated at intervals of a few minutes, guided by the patient's response, including systemic blood pressure, urine flow, frequency of ectopic activity, heart rate and (whenever possible) measurements of cardiac output, central venous pressure, and/or pulmonary capillary wedge pressure. In reported trials, the optimal infusion rates have varied from patient to patient, usually 2 to 20 mcg/kg/min but sometimes slightly outside of this range. On rare occasions, infusion rates up to 40 mcg/kg/min have been required to obtain the desired effect. Rates of infusion (mL/h) for dobutamine concentrations of 500 mcg/mL, 1000 mcg/mL, and 2000 mcg/mL necessary to attain various delivery rates of dobutamine (mcg/kg/min) for patients of different weights are given in the tables below.
Concentrations of up to 5,000 mcg/mL have been administered to humans (250 mg/50 mL). The final volume administered should be determined by the fluid requirements of the patient.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
OVERDOSAGE
Overdoses of dobutamine have been reported rarely. The following is provided to serve as a guide if such an overdose is encountered.
Signs and Symptoms - Toxicity from dobutamine is usually due to excessive cardiac ß-receptor stimulation. The duration of action of dobutamine is generally short (T1/2= 2 minutes) because it is rapidly metabolized by catechol-0-methyltranferase. The symptoms of toxicity may include anorexia, nausea, vomiting, tremor, anxiety, palpitations, headache, shortness of breath, and anginal and nonspecific chest pain. The positive inotropic and chronotropic effects of dobutamine on the myocardium may cause hypertension, tachyarrhythmias, myocardial ischemia, and ventricular fibrillation. Hypotension may result from vasodilation.
Treatment - To obtain up-to-date information about the treatment of overdose, a good resource is your certified Regional Poison Control Center. Telephone numbers of certified poison control centers are listed in the Physicians' Desk Reference (PDR). In managing overdosage, consider the possibility of multiple drug overdoses, interaction among drugs, and unusual drug kinetics in your patient.
The initial actions to be taken in a dobutamine overdose are discontinuing administration, establishing an airway, and ensuring oxygenation and ventilation. Resuscitative measures should be initiated promptly. Severe ventricular tachyarrhythmias may be successfully treated with propranolol or lidocaine. Hypertension usually responds to a reduction in dose or discontinuation of therapy.
Protect the patient's airway and support ventilation and perfusion. If needed, meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, etc.
If the product is ingested, unpredictable absorption may occur from the mouth and the gastrointestinal tract. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of dobutamine.
INDICATIONS AND USAGE
Dobutamine is indicated when parenteral therapy is necessary for inotropic support in the short-term treatment of adults with cardiac decompensation due to depressed contractility resulting either from organic heart disease or from cardiac surgical procedures. Experience with intravenous dobutamine in controlled trials does not extend beyond 48 hours of repeated boluses and/or continuous infusions.
Whether given orally, continuously intravenously, or intermittently intravenously, neither dobutamine nor any other cyclic-AMP-dependent inotrope has been shown in controlled trials to be safe or effective in the long-term treatment of congestive heart failure. In controlled trials of chronic oral therapy with various such agents, symptoms were not consistently alleviated, and the cyclic-AMP-dependent inotropes were consistently associated with increased risk of hospitalization and death. Patients with NYHA Class IV symptoms appeared to be at particular risk.
HOW SUPPLIED/STORAGE AND HANDLING
Dobutamine Injection, USP, 20 mL single dose vial contains dobutamine hydrochloride, equivalent to 250 mg dobutamine per 20 mL; ten vials per carton. NDC 0143-9141-10.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
ENDING INFORMATION
For additional information, please refer to the Package Insert for full prescribing information, available on www.hikma.com.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800 FDA-1088 or www.fda.gov/medwatch.
Manufactured by:
HIKMA FARMACÊUTICA (PORTUGAL), S.A.
Estrada do Rio da Mó, 8, 8A e 8B –
Fervença – 2705-906 Terrugem SNT, PORTUGAL
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922 USA
Document Identification Number: HK-2200-v1
1 https://www.hikma.com/newsroom/article-i3630-hikma-launches-100th-injectable-medicine-in-us-with-introduction-of-vancomycin-hydrochloride-for-injection-usp/
2 Source: IQVIA MAT April 2023, generic injectable volumes by eaches, excluding branded generics and Becton Dickinson


View original content to download multimedia:https://www.prnewswire.com/news-releases/hikma-exceeds-150-injectable-medicines-in-the-us-with-launch-of-dobutamine-injection-usp-301852377.html
SOURCE Hikma Pharmaceuticals USA Inc.