Incyte (INCY) Posts Positive Results on Opzelura From HS Study
Incyte INCY announced new results from a mid-stage study evaluating the efficacy and safety of twice-daily Opzelura (ruxolitinib cream 1.5%) in adult patients with Hurley stage 1 or 2 (mild-to-moderate) hidradenitis suppurativa (HS), a chronic, debilitating skin condition.
The phase II study enrolled 69 adult patients (age ≥ 18 years) diagnosed with Hurley stage 1 or 2 HS who have a total abscess and inflammatory nodule (AN) count of 3 to ≤ 10, with no draining tunnels at screening and baseline visits.
Results showed that the study met its primary endpoint. Patients treated with Opzelura demonstrated a significantly greater reduction in abscess and inflammatory nodule (AN) count in 1.5% compared with those who applied the vehicle control at week 16.
In addition, approximately 79.2% of on-treatment patients achieved at least a 50% reduction in AN count (AN50), 54.2% achieved a 75% reduction (AN75), 20.8% achieved a 90% reduction (AN90), and 20.8% achieved complete clearance (100% reduction, AN100), surpassing the 56.3%, 25.0%, 12.5% and 12.5% reductions, respectively, in the vehicle control group.
79.2% of patients who received Opzelura met the criteria for Hidradenitis Suppurativa Clinical Response (HiSCR), which indicates a 50% or greater reduction in AN count without an increase in abscesses or draining fistulas compared to 50.0% of patients in the vehicle control group.
Patients treated with ruxolitinib cream 1.5% showed a greater mean reduction in the International Hidradenitis Suppurativa Severity Score System (IHS4) score compared to the vehicle group.
Patients treated with ruxolitinib cream 1.5% showed a change -1.85 and -1.42 from baseline in the Skin Pain Numeric Rating Scale (NRS) and Itch NRS at Week 16, respectively, versus a -2.61 and -2.75 change from those in the vehicle control group.
These positive results reinforce the efficacy and safety profile of ruxolitinib cream.
Opzelura is a novel cream formulation of Incyte’s selective JAK1/JAK2 inhibitor Jakafi (ruxolitinib). The drug is approved by the FDA for the topical treatment of nonsegmental vitiligo in patients 12 years of age and older, and is the first and only treatment for repigmentation approved for use in the United States. It is also approved in the country for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised patients 12 years of age and older.
Sales of Opzelura came in at $337.9 million in 2023. Additional label expansion of the drug will boost sales of the drug.
Shares of Incyte have plunged 17.9% in the past year compared with the industry’s decline of 4.6%.
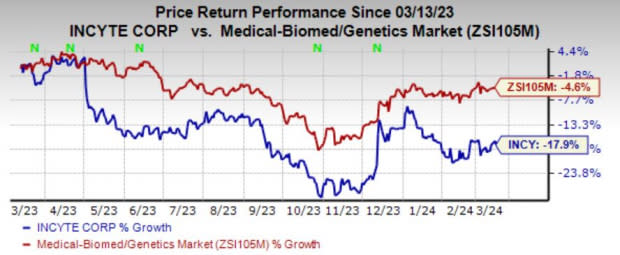
Image Source: Zacks Investment Research
Incyte missed on both earnings and sales in the fourth quarter of 2023. Lead drug Jakafi’s sales missed the top-line estimate.
Nevertheless, Opzelura’s sales beat the top-line estimate. Sales of the drug increased 78% year over year in the fourth quarter, driven by patient demand and expansion in payer coverage as the launch in atopic dermatitis and vitiligo continues.
Zacks Rank and Stocks to Consider
Incyte currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks from the drug/biotech industry are ADMA Biologics ADMA, GSK plc GSK and FibroGen FGEN. While ADMA and GSK sport a Zacks Rank #1 (Strong Buy), FGEN carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2024 earnings per share (EPS) has increased from 22 cents to 30 cents. During the same period, the estimate for ADMA’s 2025 EPS has increased from 32 cents to 50 cents. Over the past year, ADMA shares have surged 97.5%.
In the past 30 days, the Zacks Consensus Estimate for GSK’s EPS has increased 4 cents to $4.03. During the same period, the estimate for GSK 2025 EPS has increased from $4.35 to $4.39.
In the past 30 days, the Zacks Consensus Estimate for FibroGen’s 2024 loss per share has narrowed from $1.14 to $1.09. During the same period, the estimate for the company’s 2025 loss per share is pegged at 6 cents.
FGEN beat estimates in two of the trailing four quarters, missing the mark on the other two occasions, delivering an average negative surprise of 2.26%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Incyte Corporation (INCY) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
FibroGen, Inc (FGEN) : Free Stock Analysis Report
