Inspire (INSP) Gets FDA Nod for Expanded Indication of AHI
Inspire Medical Systems, Inc. INSP recently received FDA approval for the expanded indication of the company’s Apnea Hypopnea Index (“AHI”). The update includes an increase in the upper limit of the AHI to 100 events per hour from 65 and raises the Body Mass Index warning on the labeling to 40 from 32.
The latest development is likely to have strengthened Inspire Medical’s commitment to developing and commercializing innovative, minimally-invasive solutions for patients with obstructive sleep apnea (OSA).
About the Inspire Therapy
INSP’s proprietary Inspire system is the first and only FDA-approved neurostimulation technology that provides safe and effective treatment for moderate to severe OSA. The company has developed an advanced, closed-loop solution that continuously monitors a patient’s breathing and delivers mild hypoglossal nerve stimulation to maintain an open airway.
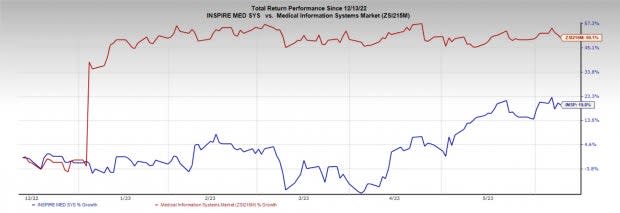
Image Source: Zacks Investment Research
Significance of the News
Patients with AHI up to 100 events per hour experience severe OSA and historically had limited treatment options available. Data from the company’s ADHERE registry demonstrated that Inspire is a safe and effective treatment for this patient population.
Industry Prospects
Per a Research report, the global sleep apnea devices market was valued at $ 4.2 billion in 2022 and is expected to witness a CAGR of 6.2% up to 2030.
OSA is a sleep disorder with irregular breathing reducing oxygen supply to the brain. Changing lifestyles, a growing geriatric population and the rising prevalence of chronic disorders are contributing to the growing incidence of OSA.
Recent Developments
In the first quarter of 2023, Inspire achieved two significant milestones.
Earlier in March, Inspire Medical received FDA approval to offer Inspire therapy to pediatric patients with Down syndrome. Inspire has been prescribed to adults with Down syndrome for several years, but previously only patients who were at least 18 years of age had access. The approval of the pediatric population with Down syndrome will include OSA patients who are at least 13 years old, with an AHI between 10 and 50 and do not have the ability to benefit from CPAP.
In the same month, INSP received countrywide reimbursement approval in Belgium, with immediate effect, at rates consistent with other countries worldwide. In 2010, Inspire therapy received European Conformity Marking for commercialization in Europe. Since then, procedures have been performed at the Antwerp University Hospital (“UZA”) in Antwerp, Belgium with funding through internal hospital budgets. This recent reimbursement approval provides a pathway to open additional Inspire implanting centers in Belgium, under the guidance of UZA while expanding access to care.
Price Performance
In the past six months, Inspire Medical shares have increased 19.8% compared with the industry’s rise of 50.1%.
Zacks Rank and Key Picks
Inspire Medical Systems currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall healthcare sector are Penumbra PEN, Lantheus LNTH and Haemonetics HAE. While Penumbra and Lantheus each sport a Zacks Rank #1 (Strong Buy), Haemonetics carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Penumbra’s stock has risen 157.6% in the past year. The Zacks Consensus Estimate for Penumbra’s earnings per share (EPS) has remained constant at $1.56 for 2023 and at $2.56 for 2024 in the past 30 days.
PEN’s earnings beat estimates in each of the trailing four quarters, the average surprise being 109.42%. In the last reported quarter, the company registered an earnings surprise of 109.09%.
The Zacks Consensus Estimate for Lantheus’ 2023 EPS has remained constant at $5.60 in the past 30 days. Shares of the company have improved 39% in the past year against the industry’s 24.5% decline.
LNTH’s earnings beat estimates in each of the trailing four quarters, the average surprise being 25.77%. In the last reported quarter, the company recorded an earnings surprise of 13.95%.
Estimates for Haemonetics’ EPS have increased from $3.29 to $3.55 for 2023 in the past 30 days. Shares of the company have increased 39% in the past year against the industry’s 24.5% decline.
HAE’s earnings beat estimates in each of the trailing four quarters, the average surprise being 12.21%. In the last reported quarter, Haemonetics delivered an earnings surprise of 13.24%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
Penumbra, Inc. (PEN) : Free Stock Analysis Report
Lantheus Holdings, Inc. (LNTH) : Free Stock Analysis Report
Inspire Medical Systems, Inc. (INSP) : Free Stock Analysis Report
