Integra (IART) Completes Enrollment in the DuraSorb IDE Study
Integra LifeSciences Holding Corporation IART recently announced the completion of patient enrolment in the DuraSorb Monofilament Mesh U.S. IDE (investigational device exemption) study. This is the first and only active, prospective, multi-center IDE study in the United States evaluating the use of a surgical matrix in two-stage breast reconstruction.
The latest development is a significant milestone for Integra to obtain pre-market approval (PMA) for DuraSorb. This is also expected to strengthen the company’s Surgical Innovation Associates business.
News in Detail
Currently, FDA’s 510(K) cleared, DuraSorb Monofilament Mesh is a bioabsorbable matrix used for the reinforcement of soft tissues where weakness exists. The purpose of the DuraSorb IDE study is to evaluate the safety and effectiveness of DuraSorb to obtain PMA for use in patients undergoing two-stage breast reconstruction. The primary follow-up period is one year after device implantation.
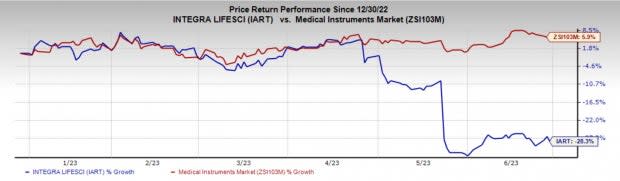
Image Source: Zacks Investment Research
The prospective multi-center study has enrolled several hundred patients from seven major academic hospitals across the country, earlier than anticipated. With the completion of the patient enrollment process, the clinical research is intended to be significant toward advancing women’s health and improving patient outcomes following breast cancer and mastectomy.
At present, IART remains the only company actively progressing toward PMAs for surgical matrices in breast reconstruction. The company is on track to file a PMA update for SurgiMend PRS with the FDA in August. Together with DuraSorb, Integra’s SurgiMend PRS will provide surgeons with two distinct soft tissue reinforcement solutions, which aim to address various clinical, contracting and economic needs across more sites of care.
Per a representative of the company, achieving these PMA breakthroughs reinforces Integra’s commitment to the implant-based breast reconstruction strategy, innovating new treatment pathways and restoring patient lives through technologies that transform surgical care.
Industry Prospects
Per a Research report, the global breast reconstruction market was valued at $465.3 million in 2021 and is expected to witness a CAGR of 6.7% by 2030.
Recent Highlights
IART’s Tissue Technologies segment has been witnessing strength in demand across its leading products including Integra Skin, MicroMatrix, Gentrix, Cytal and SurgiMend as well. Organically, the segment contributed 6.8% to the first-quarter top line. MicroMatrix’s recent launch in European markets generated positive initial feedback from wounds and reconstructive surgeons.
Last month, Integra opened a new state-of-the-art, R&D facility in Plainsboro, NJ. Dedicated to the company’s late founder Dr. Caruso, the site will be utilized in pioneering new advances in treatment pathways and setting new standards of care.
Price Performance
Shares of Integra have declined 28.3% in the past six months against the industry’s rise of 5.9%.
Zacks Rank and Key Picks
Integra currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the overall healthcare sector are Penumbra PEN, Lantheus LNTH and Haemonetics HAE. Penumbra, Lantheus and Haemonetics each sport a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Penumbra’s stock has surged 164.5% in the past year. The Zacks Consensus Estimate for Penumbra’s earnings per share (EPS) has remained constant at $1.56 for 2023 and $2.56 for 2024 in the past 30 days.
PEN’s earnings beat the consensus mark in each of the trailing four quarters, the average surprise being 109.42%. In the last reported quarter, the company registered an earnings surprise of 109.09%.
The Zacks Consensus Estimate for Lantheus’ 2023 EPS has remained constant at $5.60 in the past 30 days. Shares of the company have rallied 28% in the past year against the industry’s 20.8% decline.
LNTH’s earnings beat estimates in each of the trailing four quarters, the average surprise being 25.77%. In the last reported quarter, the company recorded an earnings surprise of 13.95%.
Estimates for Haemonetics’ EPS for 2023 have increased from $3.55 to $3.56 in the past 30 days. Shares of the company have surged 28.9% in the past year against the industry’s 20.8% decline.
HAE’s earnings beat estimates in all of the trailing four quarters, the average surprise being 12.21%. In the last reported quarter, Haemonetics delivered an earnings surprise of 13.2%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Integra LifeSciences Holdings Corporation (IART) : Free Stock Analysis Report
Haemonetics Corporation (HAE) : Free Stock Analysis Report
Penumbra, Inc. (PEN) : Free Stock Analysis Report
Lantheus Holdings, Inc. (LNTH) : Free Stock Analysis Report
