Intra-Cellular (ITCI) Q3 Earnings Top, Product Sales View Raised
Intra-Cellular Therapies, Inc. ITCI incurred a loss of 25 cents per share for the third quarter of 2023, narrower than the Zacks Consensus Estimate of a loss of 59 cents. The reported loss was also narrower than the year-ago quarter’s loss of 57 cents per share. This was due to higher product sales.
Total revenues, comprising product sales and grant revenues, came in at $126.2 million compared with $71.9 million in the year-ago period. The top line also comprehensively beat the Zacks Consensus Estimate of $118 million.
Quarter in Detail
Caplyta, the only approved drug in Intra-Cellular’s portfolio, was approved by the FDA in December 2019 for treating schizophrenia in adults. The drug also received FDA approval for treating bipolar depression in December 2021. Post this approval, Caplyta sales have increased significantly and the company expects to sustain the momentum.
Net product revenues, comprising Caplyta sales, were up almost 75% year over year to $125.8 million from $71.9 million in the prior-year period on the back of strong prescription uptake. The drug’s sales increased 14% sequentially.
Per management, Caplyta prescriptions increased 71% year over year in the third quarter of 2023 and 7% sequentially.
Research and development (R&D) expenses increased 24.9% to $41.6 million from the year-ago quarter’s figure.
Selling, general and administrative (SG&A) expenses were $105.2 million, increasing 19% year over year.
As of Sep 30, 2023, ITCI had cash, cash equivalents, restricted cash and investment securities of $494.8 million compared with $514.6 million as of Jun 30, 2023.
Shares of Intra-Cellular have lost 1% in the year-to-date period compared with the industry’s decline of 22.5%.
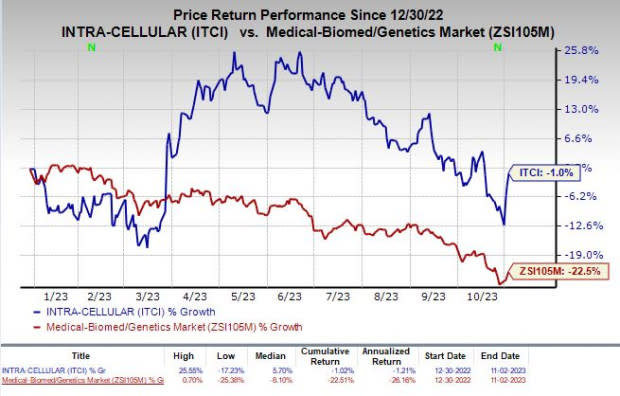
Image Source: Zacks Investment Research
2023 Guidance
Based on the strong performance of Caplyta, Intra-Cellular raised the net product sales guidance to the range of $460 million to $470 million (previously $445-$465 million) for full-year 2023.
The company, however, lowered its full-year 2023 SG&A expenses guidance to $405 million to $420 million compared with the earlier projection of $420-$450 million. The R&D expense guidance was also lowered to the range of $185-$200 million versus the previous expectation of $195-$220 million.
Pipeline Updates
Intra-Cellular is currently evaluating 42 mg of lumateperone in several late-stage studies as a treatment for major depressive disorder (MDD). The company expects top-line data from phase III Study 501 in the first quarter of 2024 and phase III Study 502 in the second quarter of 2024.
Based on the data, the company plans to file a supplemental new drug application to the FDA seeking approval for lumateperone as adjunctive therapy to antidepressants for the treatment of MDD in the second half of 2024.
Intra-Cellular Therapies Inc. Price, Consensus and EPS Surprise
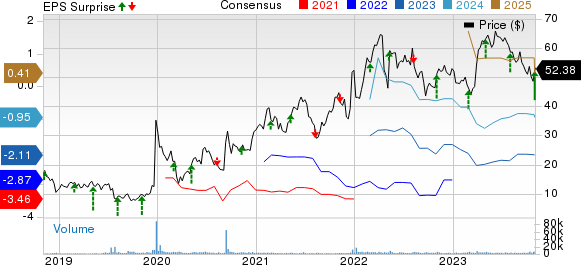
Intra-Cellular Therapies Inc. price-consensus-eps-surprise-chart | Intra-Cellular Therapies Inc. Quote
Zacks Rank & Stocks to Consider
Intra-Cellular currently carries a Zacks Rank #4 (Sell).
Some better-ranked stocks in the healthcare sector are Dynavax Technologies Corporation DVAX, MEI Pharma, Inc. MEIP and Ligand Pharmaceuticals Incorporated LGND, sporting a Zacks Rank #1 (Strong Buy) each. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Dynavax Technologies’ 2023 loss per share have narrowed from 24 cents to 22 cents. Meanwhile, during the same period, earnings per share estimates for 2024 have improved from 2 cents to 8 cents. Year to date, shares of DVAX have rallied 32.2%.
Earnings of Dynavax Technologies beat estimates in two of the last four quarters while missing the same on the remaining two occasions. DVAX delivered a four-quarter average earnings surprise of 25.78%.
In the past 60 days, estimates for MEI Pharma’s 2023 loss per share have improved from $6.54 to $4.89. During the same period, loss per share estimates for 2024 have narrowed from $5.14 to $4.02. Year to date, shares of MEIP have rallied 41.6%.
Earnings of MEI Pharma beat estimates in three of the trailing four quarters and met the same on the other occasion. On average, MEIP came up with a four-quarter earnings surprise of 53.58%.
In the past 60 days, Ligand Pharmaceuticals’ earnings per share estimates for 2023 have improved from $4.98 to $5.10. During the same period, earnings per share estimates for 2024 have moved up from $4.26 to $4.59. Year to date, shares of LGND have lost 22.4%.
Earnings of Ligand Pharmaceuticals beat estimates in three of the trailing four quarters and missed the same on the other occasion. On average, LGND came up with a four-quarter earnings surprise of 52.47%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report
MEI Pharma, Inc. (MEIP) : Free Stock Analysis Report
Intra-Cellular Therapies Inc. (ITCI) : Free Stock Analysis Report
