Ionis' (IONS) Donidalorsen Meets Late-Stage HAE Study Goal
Ionis Pharmaceuticals IONS announced positive top-line results from the phase III OASIS-HAE study on its investigational drug donidalorsen in patients aged 12 years and older with hereditary angioedema (HAE).
The OASIS-HAE study randomized study participants into three equal groups over a 24-week treatment period — two groups evaluating an 80mg dose of donidalorsen administered once every four weeks (Q4W) and another group wherein the drug was administered once every eight weeks (Q8W). The third group administered a placebo to patients once every eight weeks. All study participants will be dosed with either the drug or placebo via subcutaneous injection.
The study achieved its primary endpoint of a statistically significant reduction in the rate of HAE attacks in patients who were treated with an 80mg dose of donidalorsen once in the Q4W and Q8W groups. Though Ionis did not mention any numerical data/figures from the study, it did mention that study participants treated with donidalorsen achieved statistical significance on all secondary endpoints in the Q4W group and key secondary endpoints in the Q8W group.
Based on these results, Ionis plans to file a new drug application (“NDA”) for donidalorsen with the FDA. Management also announced that its partner Otsuka, to whom it out-licensed exclusive rights for the drug in Europe last month, is also undergoing preparations for a regulatory submission with the EMA.
Management also plans to present the above data at a future medical meeting. Ionis also expects to report data from late-stage OASIS-Plus open-label extension study in mid-2024.
A rare genetic disease, HAE is marked by severe and potentially fatal swelling of the arms, legs, face and throat.
In the past year, Ionis’ shares have risen 29.4% against the industry’s 6.9% fall.
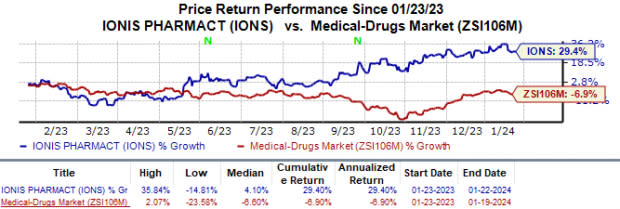
Image Source: Zacks Investment Research
Donidalorsen is one of Ionis’ wholly-owned pipeline candidates. The company has previously mentioned that it intends to launch donidalorsen independently in the United States. If approved, the drug’s commercial launch is part of management's broad strategy to deliver a steady flow of wholly-owned medicines to patients.
In November, Ionis reported positive data from the two-year analysis of an ongoing phase II open-label expansion study evaluating donidalorsen in HAE patients. Data from the study showed that treatment with the drug led to a 96% overall sustained mean reduction in HAE attack rates across all dosing groups. Per management, these results demonstrate the efficacy and safety of donidalorsen in treating HAE patients.
If approved, donidalorsen will compete with is likely to compete with BioCryst Pharmaceuticals’ BCRX Orladeyo and Takeda’s Takhzyro, both of which are approved by the FDA as prophylactic treatments for HAE attacks. While the Takeda drug is administered subcutaneously once, the BioCryst drug is administered orally once daily.
Based on the drug’s administration, donidalorsen is likely to hold an edge over Takhzyro as the latter is required to be taken once every two weeks while the former was tested for administration once every four weeks. However, the BioCryst drug is an oral pill that is required to be taken once daily as opposed to donidalorsen, which requires help for subcutaneous administration.
Like Ionis, some companies are also evaluating their own HAE candidates in clinical development. Last month, Pharvaris PHVS reported encouraging data from a mid-stage study evaluating its investigational oral drug deucrictibant for the prophylactic treatment of HAE attacks. The Pharvaris conducted study achieved its primary endpoint of the time-normalized number of investigator-confirmed HAE attacks during the 12-week treatment period. Data from the study showed an 84.5% reduction in monthly attack rate in patients who received a 40mg dose of Pharvaris’ deucrictibant compared to those receiving a placebo.
Ionis Pharmaceuticals, Inc. Price
Ionis Pharmaceuticals, Inc. price | Ionis Pharmaceuticals, Inc. Quote
Zacks Rank & A Key Pick
Ionis currently carries a Zacks Rank #2 (Buy). A better-ranked stock in the overall healthcare sector is CytomX Therapeutics CTMX, which sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for CytomX Therapeutics for 2023 have swung from a loss of 10 cents per share to earnings of 2 cents. During the same period, estimates for 2024 have narrowed from a loss of 22 cents to a loss of 6 cents. Shares of CytomX have lost 40.3% in the past year.
CytomX Therapeutics’ earnings beat estimates in three of the last four quarters while missing the estimates on one occasion. On average, the company witnessed an average surprise of 45.44%. In the last reported quarter, CytomX Therapeutics’ earnings beat estimates by 123.53%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
BioCryst Pharmaceuticals, Inc. (BCRX) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
CytomX Therapeutics, Inc. (CTMX) : Free Stock Analysis Report
Pharvaris N.V. (PHVS) : Free Stock Analysis Report
