Ionis (IONS) Drug Meets Mid-Stage MASH Study Goals
Ionis Pharmaceuticals IONS announced positive results from a phase II study on its investigational drug ION224 in adult patients with metabolic dysfunction-associated steatohepatitis (MASH) over 51 weeks.
Study participants who received 90mg and 120mg doses of ION224 achieved the primary endpoint of statistically significant liver histologic improvement. This was measured by at least a two-point reduction in the non-alcoholic fatty liver disease (NAFLD) activity score, or NAS. A subgroup analysis indicated that significant improvements in the primary endpoint were observed in patients with F2 and F3 (advanced) fibrosis.
The study also achieved a key secondary endpoint of statistically significant MASH resolution without worsening of fibrosis, as measured by biopsy. The drug was well tolerated by study participants.
The therapy also beat the placebo in terms of reducing liver steatosis (liver fat buildup). Per management, 44% of patients in the 120-mg group achieved a greater than 50% reduction in buildup compared with 3% on placebo. Ionis also revealed that 32% of patients who received this high dose also achieved at least one stage improvement in liver fibrosis without worsening steatohepatitis compared with just 12.5% who received a placebo.
Ionis will determine the following steps to advance development on ION224 and plans to present details results from this study at a future medical meeting.
Year to date, Ionis’ shares have lost 14.7% compared with the industry’s 1.6% fall.
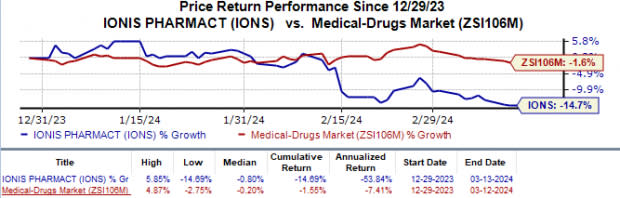
Image Source: Zacks Investment Research
Formerly called non-alcoholic steatohepatitis (NASH), MASH is the most severe form of metabolic dysfunction-associated fatty liver disease (MASLD). The disease is marked by excessive fat buildup in the liver, accompanied by inflammation and fibrosis, which may progress to cirrhosis, liver failure, cancer and death. There are no approved therapies for NASH indication.
ION224 is one of Ionis’ wholly-owned pipeline candidates. The candidate is designed to reduce the production of diacylglycerol acyltransferase 2 (DGAT2) to treat patients with MASH. A reduction in the DGAT2 enzyme reduces the overproduction of triglycerides that are responsible for the excess liver fat.
The results come just a day before the FDA announces its final decision on Madrigal Pharmaceuticals’ MDGL lead candidate in MASH indication. The regulatory filing for Madrigal’s resmetirom as a potential treatment for MASH and liver fibrosis. The filing has been granted priority review and a final decision is expected by Mar 14, 2024. If approved, the Madrigal drug will be the first approved treatment for MASH disease.
Apart from Ionis and Madrigal, several companies like Akero Therapeutics AKRO and Viking Therapeutics VKTX are also trying to develop a successful treatment.
Last week, Akero Therapeutics also reported updated results from the mid-stage HARMONY study on its own investigational lead drug efruxifermin (EFX) in MASH indication. In 2022, Akero announced that the phase IIb HARMONY study met its primary endpoint of ≥1 stage improvement in fibrosis with no worsening of MASH after 24 weeks of treatment for both the 50mg EFX (41%) and 28mg EFX (39%) dose groups compared with 20% for the placebo arm. However, in the recent data readout, it was observed that the response rates on the same primary endpoint of the HARMONY study increased to 75% for a 50mg dose of the Akero drug and 46% for a 28mg dose compared with 24% for a placebo at week 96.
Viking Therapeutics is developing a candidate, VK2809, for biopsy-confirmed NASH and fibrosis. Last year, VKTX reported positive 12-week data from the ongoing phase IIb VOYAGE study, evaluating VK2809 in patients with biopsy-confirmed NASH. The study met its primary endpoint of a statistically significant reduction in liver fat content in NASH and substantial reductions in low-density lipoprotein cholesterol (LDL-C), triglycerides and atherogenic lipoproteins against placebo. Viking Therapeutics plans to report 52-week treatment data from the VOYAGE study in the first half of 2024.
Ionis Pharmaceuticals, Inc. Price
Ionis Pharmaceuticals, Inc. price | Ionis Pharmaceuticals, Inc. Quote
Zacks Rank
Ionis currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
Madrigal Pharmaceuticals, Inc. (MDGL) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
