Ionis (IONS) Early-Stage Genetic Disorder Data Shows Promise
Ionis Pharmaceuticals IONS announced positive preliminary data from a clinical study on its pipeline candidate, ION582 (BIIB121), for the treatment of Angelman syndrome, a genetic disorder.
Ionis said that the phase I/IIa HALOS study on ION582 has completed enrolment of 51 patients at 11 global sites. Preliminary data from Part 1 of the phase I/IIa study showed encouraging electroencephalogram activity trends and early signals of positive clinical improvement. In the study. ION582 was generally well tolerated at all dose levels. The full data set from the study is expected in mid-2024. The preliminary data from the study needs to be confirmed upon analysis of the full data.
Following the encouraging data, participants from Part 1 of the study are continuing to Part 2, the long-term extension portion of the study.
It is estimated that Angelman syndrome affects an estimated one in 12,000 to 20,000 people worldwide. Angelman syndrome, which is caused by the loss of function in the UBE3A gene in the brain, is a neuro-developmental disorder that happens in early childhood and causes developmental delays, intellectual disability and sometimes seizures. At present, no treatments are approved for this disease.
So far this year, Ionis’ shares have risen 22.8% against the industry’s decrease of 16.4%.
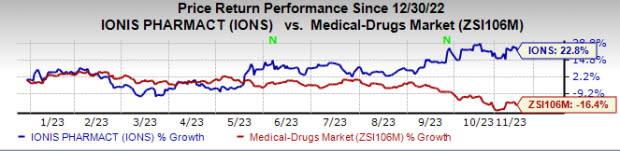
Image Source: Zacks Investment Research
Ionis is developing ION582 in partnership with Biogen BIIB.
Ionis has collaboration deals with leading drugmakers like Biogen, AstraZeneca, GSK and Novartis for developing and marketing its medicines.
Ionis has licensed Spinraza to Biogen, which is responsible for commercializing the drug approved for treating spinal muscular atrophy worldwide. Ionis receives royalties from Biogen on Spinraza’s sales.
AstraZeneca AZN, Novartis and GSK plc GSK are its partners for eplontersen, pelacarsen and bepirovirsen, respectively. Ionis and AstraZeneca’s new drug application seeking approval of eplontersen for polyneuropathy caused by hereditary TTR amyloidosis (ATTRv-PN) is under review with the FDA, with a decision expected on Dec 22, 2023. Eplontersen is also under review in the EU for the ATTRv-PN indication. Ionis and AstraZeneca are also developing eplontersen for the treatment of cardiomyopathy in the phase III CARDIO-TTRansform study. Enrollment in the study is complete and the companies are expected to report data from the study in first-half 2025.
In the third quarter, GSK announced positive data from the phase IIb B-Together study of bepirovirsen followed by pegylated interferon in patients with chronic hepatitis B virus.
Zacks Rank
Ionis currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Ionis Pharmaceuticals, Inc. Price and Consensus
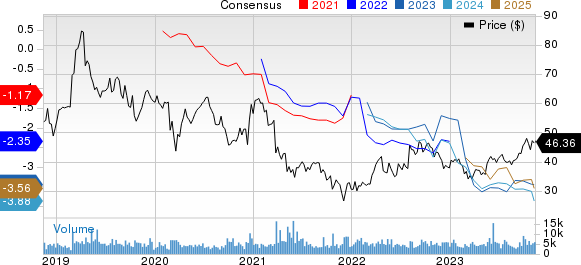
Ionis Pharmaceuticals, Inc. price-consensus-chart | Ionis Pharmaceuticals, Inc. Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
