Ionis' (IONS) Eplontersen Gets Fast Track Tag for New Indication
Ionis Pharmaceuticals, Inc. IONS announced that the FDA has granted a Fast Track designation to eplontersen for the treatment of adult patients with transthyretin-mediated amyloid cardiomyopathy (ATTR-CM).
ATTR-CM is a progressive and fatal disease that is caused by the accumulation of misfolded TTR protein in the cardiac muscle.
Ionis is developing eplontersen in partnership with British drugmaker AstraZeneca AZN.
The phase III CARDIO-TTRansform study is currently evaluating eplontersen for treating ATTR-CM. This is the largest study to be conducted in the given patient population. Data from this study is expected in 2025.
The Fast Track designation from the FDA facilitates rapid development and expedites the review of drug candidates that are being developed to treat serious conditions and for which clinical data demonstrate the potential to address unmet medical needs. The goal is to make these treatments rapidly available to patients in need.
Shares of Ionis have rallied 28.2% in the past year against the industry’s decline of 1.7%.
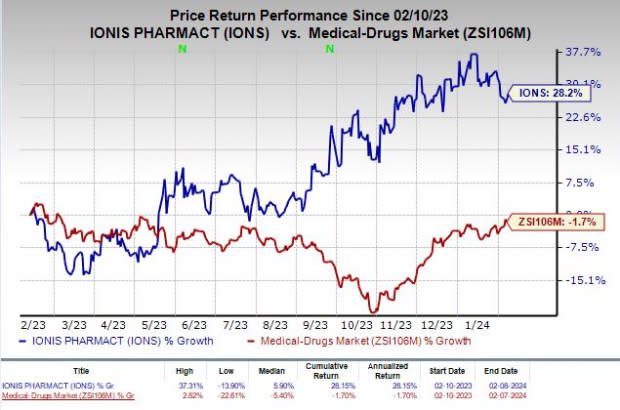
Image Source: Zacks Investment Research
Eplontersen was approved by the FDA in December 2023, under the brand name Wainua, for treating patients with hereditary transthyretin-mediated amyloid polyneuropathy, commonly called hATTR-PN or ATTRv-PN. If approved, ATTR-CM would be the second indication for eplontersen.
AstraZeneca and Ionis will market Wainua for ATTRv-PN in the United States, while AstraZeneca has exclusive rights to commercialize Wainua in ex-U.S. markets.
The commercial launch of Wainua is already underway in the United States.
Notably, regulatory applications seeking approval of eplontersen for ATTRv-PN are under review in the EU and some other countries.
A potential approval for eplontersen for the ATTR-CM indication is likely to provide a larger commercial opportunity for the product. This is because, globally, an estimated 300,000-500,000 patients are suffering from the disease.
Ionis has a broad pipeline of partnered programs with Biogen BIIB, AstraZeneca, Novartis and GSK, among other large drugmakers.
Ionis has licensed Spinraza to Biogen, which is responsible for commercializing the drug approved for treating spinal muscular atrophy (SMA) worldwide. Ionis receives royalties from Biogen on Spinraza’s sales.
BIIB has also expanded its collaboration with Ionis to identify new gene therapies for the treatment of SMA as well as a broad range of neurological diseases. Qalsody, which received accelerated approval for treating SOD-1 amyotrophic lateral sclerosis, is the second marketed drug developed under this partnership.
Zacks Rank & Stock to Consider
Ionis currently carries a Zacks Rank #3 (Hold).
A better-ranked stock in the same sector is ImmunoGen, Inc. IMGN sporting a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for ImmunoGen’s 2024 earnings per share have improved from 36 cents to 37 cents. In the past year, shares of IMGN have skyrocketed 617.9%.
Earnings of ImmunoGen beat estimates in each of the trailing last four quarters. IMGN delivered a four-quarter average earnings surprise of 136.05%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
ImmunoGen, Inc. (IMGN) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
