Ionis (IONS) Inks New Deal With Roche to Develop Novel RNA Drugs
Ionis Pharmaceuticals IONS announced that it has entered into a new collaboration agreement with pharma giant Roche RHHBY for two undisclosed, investigational RNA-based medicines for the potential treatment of Alzheimer's disease (“AD”) and Huntington's disease (“HD”).
Per the terms of the agreement, Ionis will utilize its proprietary technology platforms to discover the two RNA-based therapeutics designed to target AD and HD indications. Roche will have exclusive global rights over the two drugs developed under this agreement and will be solely responsible for their clinical development and commercialization.
In return, Ionis will receive an upfront payment of $60 million from Roche. In addition, the company will also be eligible to receive potential milestone payments and tiered royalties on the sales of future products developed under the agreement.
This new agreement with Roche extends the company’s previous agreements over the past decade. Initially, both companies entered the first collaboration in 2013 to develop tominersen for HD indication. The drug is currently being evaluated in the proof-of-concept phase II GENERATION HD2 study in people with prodromal or early manifest HD. Ionis and Roche signed another partnership deal in 2018 to develop IONIS-FB-LRx for IgA nephropathy (“IgAN”) and geographic atrophy (“GA”) indications.
In the year so far, the share price of Ionis has risen 24.8% against the industry’s 8.0% fall.
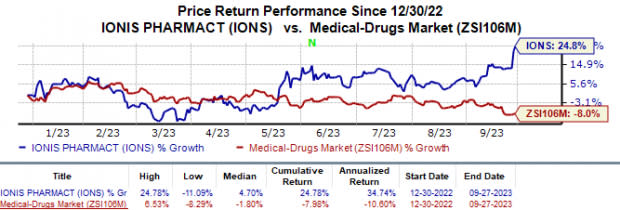
Image Source: Zacks Investment Research
Ionis intends to advance its wholly-owned pipeline development using the funds received from this expanded partnership. Earlier this week, Ionis reported positive top-line results from the phase III BALANCE study on its investigational LICA drug olezarsen people with familial chylomicronemia syndrome (“FCS”). This rare genetic disorder currently has no FDA-approved therapies. The study achieved its primary and critical secondary endpoints. Based on these results, Ionis plans to file a new drug application (“NDA”) for olezarsen with the FDA early next year.
The encouraging results from the BALANCE study highlight an essential milestone for Ionis toward its goal of launching its first commercial product. Currently, IONS earns all its commercial revenues primarily through royalty payments on net sales of Biogen BIIB-marketed drug Spinraza and R&D revenues from partnered medicines. The company has yet to generate revenues from product sales.
Apart from FCS, Ionis is also evaluating olezarsen in three ongoing phase III studies – CORE, CORE2 and ESSENCE – as a potential treatment for severe hypertriglyceridemia (“SHTG”).
Ionis’ pipeline consists of both wholly owned as well as partnered pipelines. Besides Roche, the company has partnerships with big pharma companies like AstraZeneca AZN, Biogen, and Novartis NVS.
Biogen, AstraZeneca and Novartis are Ionis’ partners for tofersen, eplontersen and pelacarsen, respectively. Last December, AstraZeneca filed an NDA with the FDA seeking approval of eplontersen for polyneuropathy caused by hereditary TTR amyloidosis (ATTRv-PN). A final decision on the NDA is expected before this year’s end. Apart from ATTRv-PN, Ionis and AstraZeneca also evaluate eplontersen as a potential treatment for amyloid transthyretin cardiomyopathy (ATTR-CM) in the phase III CARDIO-TTRansform study.
Biogen and Ionis are collaborating on developing advanced treatments for neurological disorders. Ionis has licensed Spinraza to Biogen, which is approved for treating spinal muscular atrophy in pediatric and adult patients. While Biogen is responsible for commercializing Spinraza worldwide, Ionis receives royalties on Spinraza’s sales.
The company is developing another pipeline candidate, pelacarsen, in collaboration with Novartis. Ionis and Novartis are evaluating pelacarsen in the ongoing phase III cardiovascular outcome study, HORIZON, in patients with established cardiovascular disease and elevated lipoprotein(a) or Lp(a). Novartis is responsible for leading the candidate's global development and commercialization activities.
Ionis Pharmaceuticals, Inc. Price
Ionis Pharmaceuticals, Inc. price | Ionis Pharmaceuticals, Inc. Quote
Zacks Rank
Ionis currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
