Ionis (IONS) Out Licenses Europe Rights for HAE Drug to Otsuka
Ionis Pharmaceuticals IONS signed a license agreement with Otsuka Pharmaceutical. Per the terms, Otsuka Pharmaceutical will acquire exclusive rights to market its investigational late-stage hereditary angioedema (HAE) therapy, donidalorsen, in Europe.
Per the agreement, Otsuka will be responsible for all regulatory filings and commercialization activities for the drug in Europe. Ionis will continue to maintain responsibility for the non-clinical and clinical development of donidalorsen
In consideration of granting these rights, Ionis will receive an upfront payment of $65 million and will also be eligible for milestone payments. Ionis will also be eligible to receive tiered royalties on the drug’s net sales, ranging from 20-30%.
One of Ionis’ wholly-owned pipeline candidates, donidalorsen, is being evaluated in an ongoing pivotal phase III study in HAE patients. Top-line data from this study is expected in the first half of 2024.
A rare genetic disease, HAE is marked by severe and potentially fatal swelling of the arms, legs, face and throat.
Last month, Ionis reported positive data from the two-year analysis of a phase II open-label expansion study evaluating donidalorsen in HAE patients. Data from the study showed that treatment with the drug led to a 96% overall sustained mean reduction in HAE attack rates across all dosing groups.
Year to date, Ionis’ shares have risen 34.3% against the industry’s 3.7% fall.
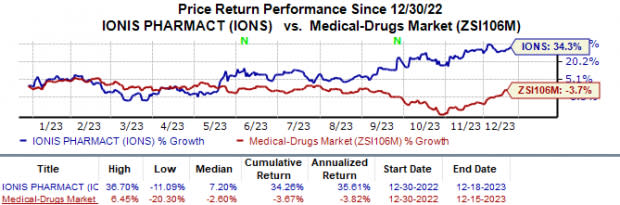
Image Source: Zacks Investment Research
Ionis intends to launch donidalorsen independently in the United States. If approved, the drug’s commercial launch is part of management's broad strategy to deliver a steady flow of wholly-owned medicines to patients.
Currently, Ionis’s pipeline consists of both partnered and wholly-owned candidates. The company has partnerships with big pharma companies like AstraZeneca AZN, Biogen BIIB, and Novartis NVS.
Biogen, AstraZeneca and Novartis are Ionis’ partners for tofersen, eplontersen and pelacarsen, respectively. Last year, AstraZeneca filed a new drug application (NDA) with the FDA seeking approval of eplontersen for polyneuropathy caused by hereditary TTR amyloidosis (ATTRv-PN). A final decision on the NDA is expected before the end of this week. Apart from ATTRv-PN, Ionis and AstraZeneca are evaluating eplontersen as a potential treatment for amyloid transthyretin cardiomyopathy (ATTR-CM) in the late-stage CARDIO-TTRansform study, which is on track for a data readout in first-half 2025.
Biogen and Ionis are developing advanced treatments for neurological disorders. Ionis licensed Spinraza to Biogen, which is approved for treating spinal muscular atrophy in pediatric and adult patients. While Biogen is responsible for commercializing Spinraza worldwide, Ionis receives royalties on Spinraza’s sales.
Ionis and Novartis are evaluating pelacarsen in the ongoing phase III cardiovascular outcome study, HORIZON, in patients with established cardiovascular disease and elevated lipoprotein(a) or Lp(a). Novartis is responsible for leading the candidate's global development and commercialization activities.
Ionis Pharmaceuticals, Inc. Price
Ionis Pharmaceuticals, Inc. price | Ionis Pharmaceuticals, Inc. Quote
Zacks Rank
Ionis currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
