Ionis (IONS) Q2 Loss Narrows Y/Y, Sales Surpass Estimates
Ionis Pharmaceuticals IONS reported a loss of 60 cents per share for second-quarter 2023, which was much narrower than the year-ago a loss of 74 cents per share.
The bottom line includes compensation expense related to equity awards. Excluding these special items, the adjusted loss per share was 41 cents per share versus loss of 56 cents per share in the year-ago quarter.
Total revenues were $188 million in the second quarter, which significantly beat the Zacks Consensus Estimate of $136.8 million. Revenues rose 40% year over year due to higher payments from partnered programs.
Quarter in Detail
Ionis has licensed Spinraza to Biogen BIIB. Biogen is responsible for commercializing Spinraza, approved for treating spinal muscular atrophy, or SMA, worldwide. Ionis receives royalties from Biogen on Spinraza’s sales.
Commercial revenues were $78 million in the second quarter, flat year over year. Commercial revenues beat our model estimate of $75.1 million as well as the Zacks Consensus Estimate of $74 million
Commercial revenues from Spinraza royalties were $61 million, up 1.6% year over. Spinraza product sales (recorded by Biogen) were flat in the quarter, being partly hurt by competitive pressure. Spinraza royalties fell shy of our model estimate of $61.4 million but beat the Zacks Consensus Estimate of $60 million.
Revenues from Tegsedi and Waylivra distribution fees were $11 million compared with $10 million in the year-ago quarter. License and royalty revenues were $6 million in the quarter compared with $8 million in the year-ago quarter. The commercial revenues included royalty revenues from Biogen for Qalsody/tofersen, which was approved by the FDA in April for treating superoxide dismutase 1-amyotrophic lateral sclerosis (SOD1-ALS).
R&D revenues almost doubled to $110 million from the year-ago revenues of $56 million due to rapid advancement in numerous partnered programs. The R&D revenues in the quarter included a $40 million payment from AstraZeneca AZN (including $20 million for costs shared for eplontersen development) and a $16 million milestone payment from Biogen for Qalsody U.S. approval.
Adjusted operating costs were up 29.2% year over year to $252 million in the quarter, mainly due to higher R&D costs, as the company rapidly advanced its wholly-owned late-stage pipeline and increased go-to-market activities for eplontersen, olezarsen and donidalorsen.
2023 Guidance
Ionis reaffirmed its previously issued financial guidance for 2023. The company expects total revenues to be more than $575 million in 2023. Its adjusted operating loss is expected to be less than $425 million.
Adjusted operating expense is expected to be in the range of $970-$995 million. Adjusted operating costs are expected to gradually increase in the second half. R&D costs are expected to increase in the range of 20-25% year over year in 2023. SG&A costs are expected to increase approximately $35 million year over year.
The company expects its cash and investment to be approximately $2 billion in 2023.
So far this year, Ionis’ shares have risen 5.9% against the industry’s decrease of 1.7%.
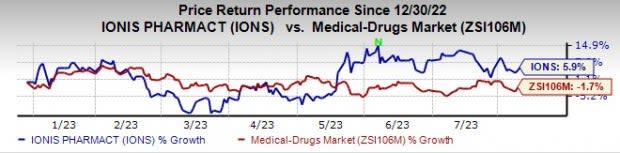
Image Source: Zacks Investment Research
Pipeline Update
Ionis has phase III studies ongoing for eight medicines (internal as well as partnered) across 10 indications.
Some of these candidates are pelacarsen for cardiovascular disease due to elevated Lp(a) levels, olezarsen for familial chylomicronemia syndrome (FCS) and severe hypertriglyceridemia, eplontersen for TTR amyloidosis, ION363 for ALS, with mutations in the fused in sarcoma gene, or FUS; donidalorsen for hereditary angioedema and bepirovirsen for chronic hepatitis B.
AstraZeneca, Novartis NVS and GSK are its partners for eplontersen, pelacarsen and bepirovirsen, respectively. Ionis and AstraZeneca’s new drug application seeking approval of eplontersen for polyneuropathy caused by hereditary TTR amyloidosis (ATTRv-PN) is under review with the FDA with a decision expected on Dec 22, 2023.
Last month, Ionis announced longer term week 85 data from the NEURO-TTRansform study, which showed that a substantial number of patients treated with eplontersen continued to demonstrate improvement in neuropathy impairment and quality of life through the 85 weeks of treatment, highlighting eplontersen's durable efficacy.
Ionis and AstraZeneca are also developing eplontersen for the treatment of cardiomyopathy in the phase III CARDIO-TTRansform study. In July, Ionis announced that enrollment in the study is complete and the companies expect to report data in the first half of 2025.
Roche recently initiated phase IIII development of IONIS-FB-LRx in IgA nephropathy.
Building on the existing collaboration with Novartis for pelacarsen, earlier this month, Ionis announced a new collaboration with Novartis to create a next-generation compound targeting lipoprotein(a), or Lp(a) for cardiovascular disease as a potential follow-on to pelacarsen. For the deal, Ionis will get an upfront payment of $60 million from Novartis.
Pelacarsen is currently being evaluated in a phase III cardiovascular outcome study with data expected in 2025.
Ionis is advancing and expanding its wholly-owned pipeline to drive future revenue growth. Many of these candidates — olezarsen, ION-363 and donidalorsen — are being evaluated in late-stage studies. Data from the phase III BALANCE study of olezarsen in FCS is expected in the second half of this year. Data from the phase III OASIS-HAE study evaluating donidalorsen in preventing angioedema attacks is expected in the first half of 2024.
Zacks Rank
Ionis currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Ionis Pharmaceuticals, Inc. Price and Consensus
Ionis Pharmaceuticals, Inc. price-consensus-chart | Ionis Pharmaceuticals, Inc. Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Biogen Inc. (BIIB) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
