Iovance (IOVA) Up Despite FDA's Delayed Decision on Melanoma Drug
Iovance Biotherapeutics IOVA announced that the FDA has postponed the previously expected decision date, regarding the company’s biologics license application (BLA) for lifileucel, from Nov 25, 2023 to Feb 24, 2024. This delay is due to the FDA’s resource constraints, which are affecting the priority review of the lifileucel BLA.
The BLA is seeking accelerated approval of lifileucel for patients with advanced melanomawho progressed on or after prior anti-PD-1/L1 therapy. Currently, there are no FDA-approved therapies in this treatment setting.
Per Iovance, the regulatory body requires additional time to complete the priority review of its lifileucel BLA. The FDA does not have time and resources to review the response to a query that Iovance had given before a meeting scheduled on Sep 11, 2023. However, the FDA reassured IOVA to work closely with the company to expedite the remaining review for a potentially earlier approval date.
Also, the extension of the PDUFA date does not impact the Priority Review status or the previously granted Regenerative Medicine Advanced Therapy designation of lifileucel.
Iovance’s lead product candidate, lifileucel, is a novel therapy based on the company’s proprietary tumor infiltrating lymphocyte (TIL) technology. Subject to approval, lifileucel will become the first and only TIL therapy for advanced melanoma patients, as well as the first one-time cell therapy for a solid tumor cancer.
The regulatory body has reiterated the absence of any major review issues with the BLA and also stated that there are no plans to hold an advisory committee meeting. Furthermore, the FDA has also successfully completed all pre-approval inspections of clinical sites, internal and external manufacturing and testing facilities.
The stock of the company rose 14.1% in the after-market hours on Thursday despite the delay in FDA’s decision date for lifileucel BLA. Year to date, shares of Iovance have lost 27.3% compared with the industry’s 13.8% fall.
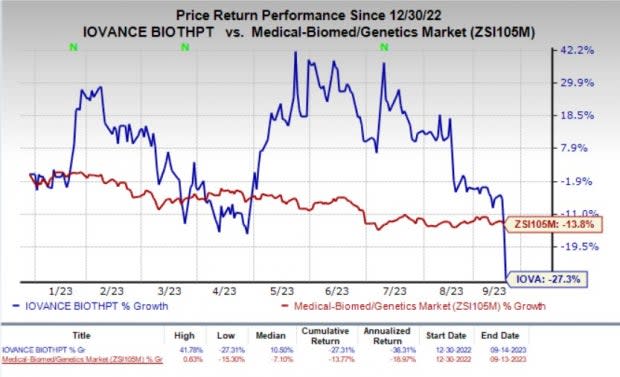
Image Source: Zacks Investment Research
The BLA filing, completed earlier this March, is based on data from cohorts 2 and 4 of the phase II C-144-01 study, which evaluated lifileucel in patients with post-anti-PD1 melanoma. Overall data from these cohorts shows that treatment with lifileucel achieved an objective response rate (ORR) of 31%, with the median duration of the response still not reached at 36.5 months.
IOVA is also evaluating lifileucel in a late-stage study in combination with Merck’s MRK Keytruda (pembrolizumab), against Keytruda monotherapy, in frontline patients with advanced (unresectable or metastatic) melanoma.
The phase III TILVANCE-301 study is being conducted in two arms, seeking to randomize approximately 670 patients. The investigational late-stage study will evaluate the safety and efficacy of lifileucel in combination with Merck’s Keytruda (experimental arm) compared with Keytruda as a monotherapy (control arm). The study is currently enrolling patients with advanced melanoma who have not received prior therapy for advanced disease. The dual primary endpoints of the TILVANCE-301 study are ORR and progression-free survival (PFS).
In June 2023, the company announced randomizing the first patient in its phase III TILVANCE-301 study of the combo therapy of lifileucel and MRK’s Keytruda.
Per a previous agreement with the FDA, the dual primary endpoints of ORR and PFS in the TILVANCE-301 can support accelerated and full approvals of the lifileucel/Keytruda combo in frontline advanced melanoma.
In the latest press release, Iovance reported that the FDA remains engaged and has not expressed any concerns about the status of the TILVANCE-301 confirmatory study in frontline advanced melanoma. The TILVANCE-301 study is expected to be well underway by the time of the extended PDUFA date for lifileucel BLA in post-anti-PD-1 melanoma.
Thus, in the event of potential accelerated approval of lifileucel in post-anti-PD-1 melanoma, the FDA has additionally agreed that the TILVANCE-301 study can simultaneously serve as the confirmatory study for full approval in the initial monotherapy indication.
Iovance Biotherapeutics, Inc. Price and Consensus
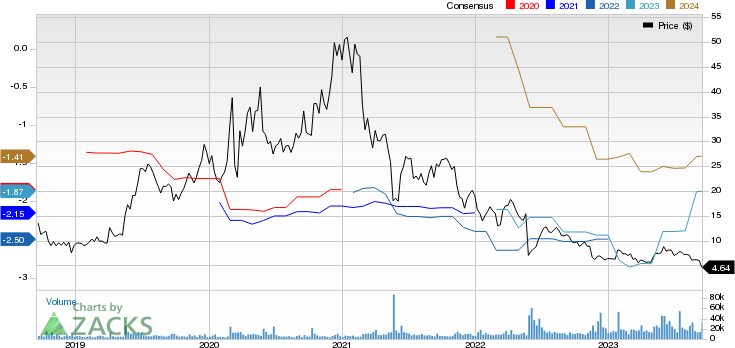
Iovance Biotherapeutics, Inc. price-consensus-chart | Iovance Biotherapeutics, Inc. Quote
Zacks Rank and Stocks to Consider
Iovance currently has a Zacks Rank #3 (Hold).
A few better-ranked stocks in the same industry are Dynavax Technologies DVAX and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present.
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 30 days, the Zacks Consensus Estimate for Dynavax’s 2023 loss per share has remained constant at 24 cents. The estimate for Dynavax’s 2024 earnings per share is currently pegged at 2 cents. Year to date, shares of DVAX have risen by 32.8%.
DVAX’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 25.78%.
In the past 30 days, the Zacks Consensus Estimate for Corcept’s 2023 earnings per share has gone up from 75 cents to 78 cents. The estimate for Corcept’s 2024 earnings per share has also improved from 81 cents to 83 cents. Year to date, shares of CORT have climbed 66.4%.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Iovance Biotherapeutics, Inc. (IOVA) : Free Stock Analysis Report
