J&J (JNJ) to End Pulmonary Hypertension Study on Opsumit
Johnson & Johnson JNJ announced that it is stopping a phase III study called MACiTEPH, evaluating its pulmonary arterial hypertension (PAH) drug, macitentan (75 mg) in patients with chronic thromboembolic pulmonary hypertension (CTEPH).
CTEPH is a rare form of pulmonary hypertension that occurs when there is abnormally high pressure in the lungs’ small blood vessels.
The decision to end the CTEPH study, due to futility, was made at the recommendation of the study’s independent data monitoring committee following a pre-planned interim analysis.
J&J markets macitentan as Opsumit to treat PAH, WHO Group 1. PAH means there is high blood pressure in the arteries of the lungs.
J&J entered the PAH drugs category with the 2017 acquisition of Actelion. J&J’s another key PAH drug is Uptravi. J&J is also conducting a phase III study (UNISUS) to demonstrate the superiority of a 75 mg dose of Opsumit over the currently marketed 10 mg dose.
In addition, J&J’s new drug application seeking approval for the single tablet combination therapy of macitentan and tadalafil for long-term treatment of PAH is under review in the United States and EU. The regulatory applications were based on positive data from J&J’s phase III A DUE study.
J&J’s stock has declined 10.6% so far this year against an increase of 6.5% for the industry.
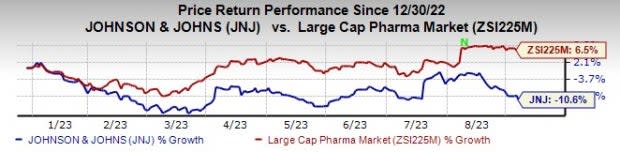
Image Source: Zacks Investment Research
In a separate press release, J&J announced positive top-line data from a phase III study evaluating regimens based on its new cancer drug, Rybrevant, in the broader EGFR-mutated non-small cell lung cancer (NSCLC) population,
The study, called MARIPOSA-2, evaluated Rybrevant plus chemotherapy with and without lazertinib versus chemotherapy alone in patients with EGFR-mutated NSCLC after disease progression on osimertinib. The study met its dual primary endpoint, resulting in statistically significant and clinically meaningful improvement in progression-free survival versus chemotherapy alone in both experimental treatment arms.
Rybrevant is presently approved for patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations whose disease progressed on or after platinum-based chemotherapy.
Another phase III study called PAPILLON has earlier demonstrated the potential of Rybrevant to improve outcomes in patients with EGFR-mutated NSCLC. PAPILLON evaluated Rybrevant in combination with chemotherapy (carboplatin-pemetrexed) in patients with newly diagnosed advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.
Last month, J&J filed a supplemental biologics license application (sBLA) to the FDA seeking approval of Rybrevant in combination with chemotherapy for first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations based on data from the PAPILLON study.
Rybrevant is being evaluated in multiple clinical studies in NSCLC, including the pivotal phase III study called MARIPOSA, which is evaluating Rybrevant and lazertinib for first-line EGFR-mutated NSCLC.
Zacks Rank & Stocks to Consider
J&J currently has a Zacks Rank #4 (Sell).
Johnson & Johnson Price and Consensus
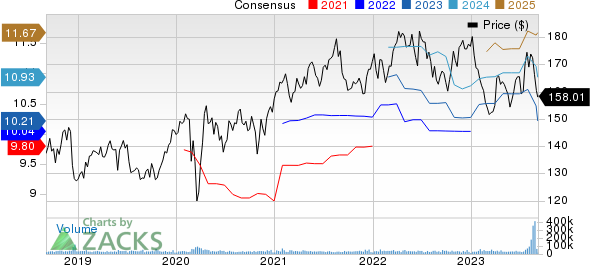
Johnson & Johnson price-consensus-chart | Johnson & Johnson Quote
Some better-ranked biotech companies are Exelixis EXEL, Dynavax Technologies Corporation DVAX and Corcept Therapeutics CORT, each carrying a Zacks Rank of 2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, estimates for Exelixis’ 2023 earnings per share have risen from 89 cents to 98 cents per share. During the same period, earnings per share estimates for 2024 have risen from $1.31 to $1.36. Year to date, shares of Exelixis have gained 38.5%.
Earnings of Exelixis beat estimates in three of the last four quarters, delivering an earnings surprise of 18.62%, on average.
In the past 60 days, estimates for Dynavax Technologies’ 2023 loss per share have narrowed from 56 cents to 24 cents, while those for 2024 have improved from a loss of 24 cents to earnings of 2 cents. Shares of Dynavax Technologies have gained 30.5% YTD.
Earnings of Dynavax Technologies beat estimates in two of the last four quarters and missed the mark on two occasions. On average, the company witnessed an earnings surprise of 25.78% over the trailing four quarters.
In the past 60 days, the Zacks Consensus Estimate for Corcept’s earnings has gone up from 62 cents per share to 78 cents for 2023. The bottom-line estimate has also improved from 61 cents to 83 cents for 2024 during the same time frame. Shares of the company have rallied 55.3% year to date.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dynavax Technologies Corporation (DVAX) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Exelixis, Inc. (EXEL) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
