KalVista (KALV) Surges 15% on Update From Angioedema Study
KalVista Pharmaceuticals, Inc. KALV announced that it has achieved its enrolment target of 114 patients in the phase III KONFIDENT study evaluating its lead product candidate, sebetralstat, for the treatment of hereditary angioedema (HAE) attacks. Following the completion of enrolment, management expects to report top-line results from the study before this year’s end.
Sebetralstat is being developed as an oral on-demand therapy for acute HAE attacks. Subject to the success of the KONFIDENT study on sebetralstat, the company also plans to submit a new drug application (NDA) to the FDA in the first half of 2024 to treat HAE attacks.
The stock of the company swiftly jumped about 15% on Friday in response to the positive updates on sebetralstat in HAE. Currently, the company has no marketed drugs in its portfolio. A potential success in developing sebetralstat for HAE will help KALV in securing approval for its first-ever marketed drug. It would also help the company in generating a stable stream of revenues, thereby enabling it to support its business operations.
Year to date, the stock has shot up 52.2% against the industry’s 4.8% decline.
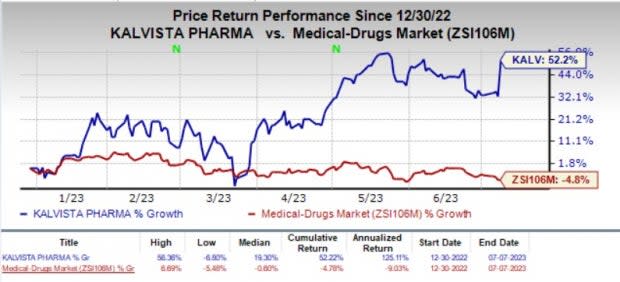
Image Source: Zacks Investment Research
KalVista also reported initiating a KONFIDENT-S two-year open-label extension (OLE) study on sebetralstat. This OLE study will evaluate the long-term safety and tolerability profile of sebetralstat. Furthermore, it will also examine sebetralstat’s potential in the treatment of short-term prophylaxis in medical and dental procedures.
We would also like to remind the investors that earlier this year, the company reported receiving FDA regulatory guidance for the oral disintegrating tablet (ODT) formulation of sebetralstat. This confirmed the requirements to support a supplemental NDA (sNDA) filing for the ODT formulation of the candidate. Per the FDA’s feedback, no additional efficacy studies with the ODT formulation will be required to support a sNDA application.
In the United States and EU, KalVista anticipates that the ODT formulation will follow the launch of the original oral formulation of sebetralstat. However, the ODT formulation of sebetralstat might become the initial launch formulation in other countries.
In the same press release, KALV reported its financial performance for the fourth quarter of fiscal 2023 and the full fiscal year 2023. In the reported quarter, KalVista reported a loss per share of 77 cents, which is narrower than the Zacks Consensus Estimate of a loss of 92 cents per share. In the year-ago quarter, the company reported a loss of 98 cents per share.
For the fiscal year 2023, KalVista reported a loss per share of $3.33, which is narrower than the loss per share of $3.36 reported in the last fiscal year.
KalVista Pharmaceuticals, Inc. Price and Consensus

KalVista Pharmaceuticals, Inc. price-consensus-chart | KalVista Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
KalVista currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks from the same industry are Akebia Therapeutics AKBA, Aurinia Pharmaceuticals AUPH and AlloVir ALVR, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Akebia Therapeutics’ 2023 loss per share has narrowed from 45 cents to 28 cents. During the same period, the estimate for AKBA’s 2024 loss per share has narrowed from 25 cents to 16 cents. Year to date, shares of the company have gained by 76.8%.
AKBA beat estimates in three of the trailing four quarters and missed the mark on one occasion, delivering an average earnings surprise of 41.41%.
In the past 90 days, the Zacks Consensus Estimate for Aurinia Pharmaceuticals’ 2023 loss per share has narrowed from 76 cents to 71 cents. During the same period, the estimate for AUPH’s 2024 loss per share has narrowed from 54 cents to 44 cents. Year to date, shares of the company have rallied by 138.2%.
AUPH beat estimates in three of the trailing four quarters and missed the mark on one occasion, delivering an average earnings surprise of 27.20%.
In the past 90 days, the Zacks Consensus Estimate for AlloVir’s 2023 loss per share has narrowed from $1.90 to $1.80. During the same period, the estimate for ALVR’s 2024 loss per share has narrowed from $1.98 to $1.89. Year to date, shares of the company have plunged 37.5%.
ALVR beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 9.78%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Akebia Therapeutics, Inc. (AKBA) : Free Stock Analysis Report
Aurinia Pharmaceuticals Inc (AUPH) : Free Stock Analysis Report
KalVista Pharmaceuticals, Inc. (KALV) : Free Stock Analysis Report
AlloVir, Inc. (ALVR) : Free Stock Analysis Report
