Krystal's (KRYS) Dermatology Drug Gets FDA Nod, Stock Up 10%
Krystal Biotech, Inc. KRYS announced the FDA approval of Vyjuvek for the treatment of patients six months of age or older with dystrophic epidermolysis bullosa (“DEB”). Notably, DEB is a rare genetic disorder that is caused by one or more mutations in the COL7A1 gene and results in the deficiency of functional type VII collagen (“COL7”) protein.
The lack of this protein in DEB patients causes extremely fragile skin that blisters and tears with minor friction or trauma. DEB patients suffer from open wounds, which lead to recurrent skin infections and fibrosis that can cause the fusion of fingers and toes, and in the worst-case scenario, increase the risk of developing an aggressive form of skin cancer.
Vyjuvek is a topical gel, which addresses the genetic root cause of DEB by delivering functional copies of the human COL7A1 gene to provide wound healing and sustained functional COL7 protein expression with redosing. Vyjuvek is the first-ever redosable gene therapy and currently forms the first and only medicine approved by the FDA for the treatment of DEB, both recessive and dominant.
Krystal shares jumped about 10% on Friday, following the positive news. In the past year, shares of the company have shot up 66.4% against the industry’s 6.4% fall.
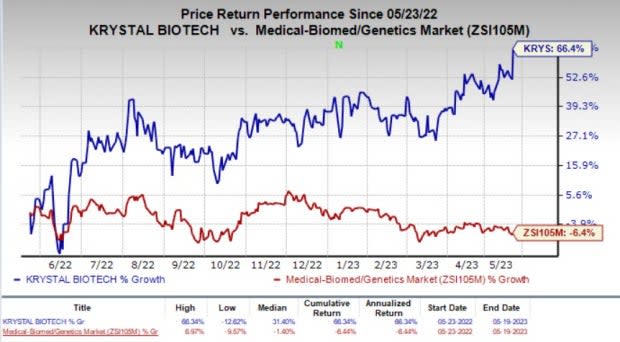
Image Source: Zacks Investment Research
The FDA approval of Vyjuvek was based on positive results from two studies, the GEM-1/2 study and the GEM-3 study. The GEM-1/2 study demonstrated a favorable effect of repeated topical application of Vyjuvek.
The subsequent GEM-3 study of Vyjuvek also met its primary endpoint of complete wound healing at six months and its key secondary endpoint of complete wound healing at three months. The candidate was reported well tolerated, with no drug-related serious adverse events or discontinuations due to treatment-related events.
Krystal anticipates the commercial launch of Vyjuvek in the United States in the third quarter of 2023.
In the same press release, Krystal also announced that the FDA has also granted a Rare Pediatric Disease Priority Review Voucher (PRV) to the company. This PRV will grant priority review to a subsequent drug application that would not otherwise qualify for priority review.
Krystal is also currently seeking approval for Vyjuvek in regions outside the United States. In Europe, Vyjuvek enjoys orphan drug designation and Priority Medicine eligibility for the treatment of DEB. The company expects to start the marketing authorization application for commercial rights in the European Union in the second half of 2023 with a potential approval in 2024.
Krystal Biotech, Inc. Price and Consensus
Krystal Biotech, Inc. price-consensus-chart | Krystal Biotech, Inc. Quote
Zacks Rank and Stocks to Consider
Krystal Biotech currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Allogene Therapeutics ALLO, Anixa Biosciences ANIX and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Allogene Therapeutics’ 2023 loss per share has narrowed from $2.83 to $2.32. In the past year, shares of Allogene Therapeutics have fallen by 18.5%.
ALLO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 5.08%.
In the past 90 days, the Zacks Consensus Estimate for Anixa Therapeutics’ 2023 loss per share has narrowed from 62 cents to 43 cents. In the past year, shares of Anixa Therapeutics have increased by 5.5%.
ANIX beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 24.04%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 19 cents to 9 cents. In the past year, shares of ADMA Biologics have increased by 98.1%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Krystal Biotech, Inc. (KRYS) : Free Stock Analysis Report
ANIXA BIOSCIENCES INC (ANIX) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report
