Kymera (KYMR) Up on Positive Data From Phase I Study on KT-474
Kymera Therapeutics, Inc. KYMR announced positive clinical data from a phase I study evaluating its highly selective, orally bioavailable IRAK4 degrader, KT-474, for the treatment of patients with hidradenitis suppurativa (“HS”) and atopic dermatitis (“AD”).
The company has a collaboration with French pharma giant Sanofi SNY for developing KT-474 outside the oncology and immune-oncology field.
Data from the above-mentioned study showed that treatment with KT-474 led to robust IRAK4 knockdown in blood and active skin lesions, as well as systemic suppression of proinflammatory cytokines and chemokines in patients with HS and AD.
Treatment with KT-474 was generally safe and well-tolerated, with no serious adverse side effects being reported.
Sanofi is looking to advance the development of KT-474 into phase II clinical studies and has notified KYMR about the same. The phase II study will initially evaluate the potential of KT-474 in HS and AD, with the first study expected to begin in 2023.
Shares of Kymera gained 14.6% on Wednesday, following the announcement of the news. However, the stock has plunged 53.3% so far this year compared with the industry’s decline of 17.6%.
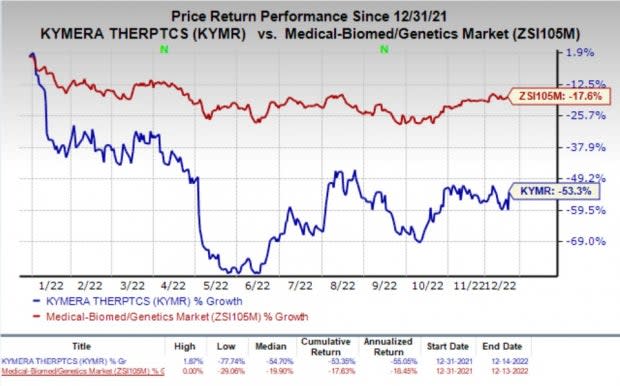
Image Source: Zacks Investment Research
Apart from this, Kymera is developing oncology degraders namely, KT-333, KT-413 and KT-253.
KT-333 is being developed for the treatment of STAT3-dependent hematological malignancies and solid tumors. A phase I study is evaluating weekly doses of KT-333 for treating adult patients with relapsed and/or refractory lymphomas, leukemias and solid tumors.
Another phase I study is investigating KT-413 for treating adult patients with relapsed and/or refractory B-cell non-Hodgkin's lymphomas.
Additional data from both studies are expected in 2023.
Meanwhile, the FDA cleared the investigational new drug application for KT-253, a degrader that targets MDM2. A phase I study evaluating KT-253 in adult patients with liquid and solid tumors is expected to begin in 2023.
We note that Kymera’s top line currently comprises collaboration revenues from SNY, as well as other healthcare companies. In absence of a marketed product, the successful development of its pipeline candidates remains in key focus for the company.
Zacks Rank & Stocks to Consider
Kymera currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are ASLAN Pharmaceuticals Limited ASLN and Aeglea BioTherapeutics, Inc. AGLE, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Loss per share estimates for ASLAN Pharmaceuticals have narrowed 6.1% for 2022 and 5.7% for 2023 in the past 60 days.
Earnings of ASLAN Pharmaceuticals surpassed estimates in two of the trailing four quarters and missed on the remaining two occasions. ASLN witnessed an earnings surprise of 1.64% on average.
Loss per share estimates for Aeglea BioTherapeutics have narrowed 7.3% for 2022 and 1.2% for 2023 in the past 60 days.
Earnings of Aeglea BioTherapeutics surpassed estimates in two of the trailing four quarters and missed on the remaining two occasions. AGLE witnessed an earnings surprise of 3.60% on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Aeglea BioTherapeutics, Inc. (AGLE) : Free Stock Analysis Report
ASLAN Pharmaceuticals Ltd. (ASLN) : Free Stock Analysis Report
Kymera Therapeutics, Inc. (KYMR) : Free Stock Analysis Report
