Kymera (KYMR) Rises as Dosing Begins in AD Study on KT-474
Kymera Therapeutics, Inc. KYMR announced that the first patient had been dosed in the phase II ADVANTA study evaluating KT-474 (SAR444656) for treating atopic dermatitisAD, also known as eczema.
Following the start of patient dosing, Kymera received a milestone payment of $15 million from French pharma giant Sanofi SNY as both companies collaborated to develop KT-474.
Shares of Kymera gained 7.2% on Dec 7 following the announcement of the news.
Sanofi is conducting the phase II study, which is evaluating the safety and efficacy of KT-474 versus placebo in adult patients with moderate to severe AD.
Kymera’s KT-474, a highly selective, orally bioavailable IRAK4 degrader, is also being developed for the treatment of hidradenitis suppurativa (“HS”).
In October 2023, SNY dosed the first patient in another phase II study evaluating KT-474 for treating HS. KYMR received a milestone payment of $40 million upon dosing of the first patient in the HS study.
Top-line data from both HS and AD studies are expected in the first half of 2025.
Shares of Kymera have lost 5.1% so far this year compared with the industry’s decline of 20%.
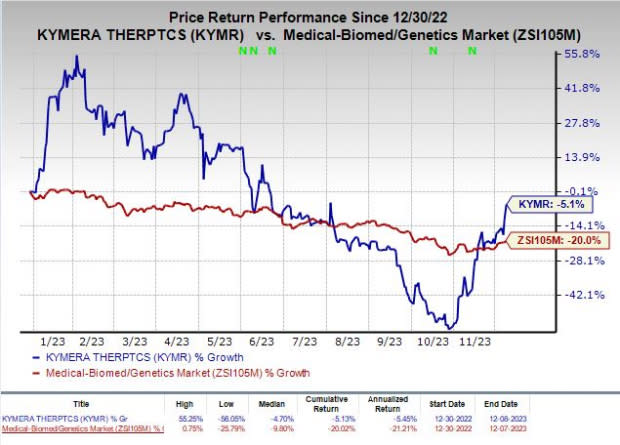
Image Source: Zacks Investment Research
Apart from this, Kymera is developing oncology degraders, namely KT-333 and KT-253.
KT-333 is being developed in early-stage studies for the treatment of cutaneous T-cell lymphoma (“CTCL”) and peripheral T-cell lymphoma (“PTCL”).
The FDA granted Fast Track designation to KT-333 for the treatment of both relapsed/refractory CTCL and relapsed/refractory PTCL in the third quarter of 2023.
The company is developing KT-253, a degrader that targets MDM2 in early-stage studies for treating patients with solid tumors and lymphomas.
We note that Kymera’s top line currently comprises collaboration revenues from Sanofi. In the absence of a marketed product, the successful development of its pipeline candidates remains a key focus for the company.
Zacks Rank & Stocks to Consider
Kymera currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the healthcare sector are Entrada Therapeutics, Inc. TRDA and Puma Biotechnology, Inc. PBYI, each sporting a Zacks Rank #1 (Strong Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Entrada Therapeutics’ 2023 loss per share have narrowed from $2.07 to 9 cents. Meanwhile, loss per share estimates for 2024 have narrowed from $2.35 to $2.04. Year to date, shares of TRDA have decreased 1.1%.
Earnings of Entrada Therapeutics beat estimates in three of the last four quarters while missing the same on the remaining occasion. TRDA delivered a four-quarter average earnings surprise of 70.68%.
In the past 60 days, estimates for Puma Biotechnology’s 2023 earnings per share have improved from 67 cents to 72 cents. During the same period, earnings per share estimates for 2024 have moved up from 55 cents to 64 cents. Year to date, shares of PBYI have lost 1.2%.
Earnings of Puma Biotechnology beat estimates in three of the last four quarters while missing the same on the remaining occasion. PBYI delivered a four-quarter average earnings surprise of 76.55%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Sanofi (SNY) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
Kymera Therapeutics, Inc. (KYMR) : Free Stock Analysis Report
Entrada Therapeutics, Inc. (TRDA) : Free Stock Analysis Report
