Lava (LVTX) Discontinues Hematological Tumor Study, Shares Fall
Lava Therapeutics LVTX announced discontinuing its ongoing phase I/IIa study on LAVA-051, which is being evaluated for multiple hematological tumors, including multiple myeloma (MM), chronic lymphocytic leukemia (CLL) and acute myeloid leukemia.
The phase I/IIa study on LAVA-051 was evaluating the candidate’s safety, tolerability, pharmacokinetics, pharmacodynamics, immunogenicity and preliminary anti-tumor activity in patients with relapsed or refractory CLL and MM.
Lava subsequently reported that the decision to discontinue the LAVA-051 study is due to a recent review of the competitive landscape that has demonstrated significant advancements, already in place, in the treatment of MM and CLL. The company further reiterated that the decision was not based on the candidate’s safety concerns.
Following this dismal news, the company’s stock dropped about 10% on Wednesday. Year to date, shares of LVTX have plunged 38.6% compared with the industry’s 6.3% fall.
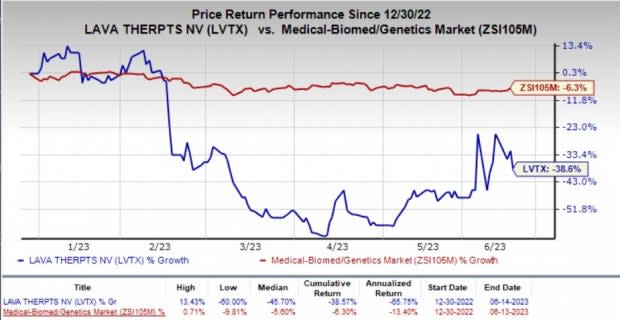
Image Source: Zacks Investment Research
Following the decision to discontinue the LAVA-051 program, Lava shall now focus its resources on LAVA-1207, a Gammabody designed to treat prostate cancer, current partnered programs and pipeline as part of its reprioritization strategy. LAVA-1207 is currently being studied in a phase I/IIa dose escalation study to treat patients with therapy refractory metastatic castration-resistant prostate cancer (mCRPC). Patient enrollment in the phase I/IIa study is currently ongoing in the United States and the EU.
The company further reported that LAVA-1207 has thus far demonstrated a favorable safety profile as well as preliminary signs of anti-tumor activity with disease stabilization and prostate-specific antigen reduction during dose escalation in the study’s heavily pretreated patient population. Management believes that immunotherapies still lack in the effective treatment of mCRPC creating a significant unmet need for new therapies in patients who have progressed on currently approved treatments.
The company expects that discontinuation of the LAVA-051 study and focusing its resources on the LAVA-1207 program will result in cost savings that will extend its cash runway further into 2026.
Lava’s collaborations include a license agreement with Seagen SGEN to develop, manufacture and commercialize SGN-EGFRd2 (LAVA-1223), which was signed in September 2022. LAVA-1223 is based on Lava’s proprietary Gammabody technology to target EGFR-expressing solid tumors.
Per the terms of the licensing agreement, Lava received a $50 million nonrefundable upfront payment in October 2022 from Seagen. Lava is further eligible to receive up to approximately $650 million upon achieving certain developmental, regulatory and commercial milestones, as well as tiered royalties ranging from the single digits to the mid-teens on future sales. On the other hand, Seagen enjoys the opportunity to exclusively negotiate rights to apply Lava’s Gammabody platform on up to two additional tumor targets.
LAVA Therapeutics N.V. Price and Consensus
LAVA Therapeutics N.V. price-consensus-chart | LAVA Therapeutics N.V. Quote
Zacks Rank and Stocks to Consider
Lava currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the biotech sector are Adaptimmune Therapeutics ADAP and Akero Therapeutics AKRO, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has remained stable at 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from 74 cents to 56 cents. Year to date, shares of ADAP have fallen by 28.8%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $3.46 to $2.80. During the same period, the estimate for Akero Therapeutics’ 2024 loss per share has narrowed from $3.66 to $3.27. Year to date, shares of AKRO have gained by 0.7%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Seagen Inc. (SGEN) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
LAVA Therapeutics N.V. (LVTX) : Free Stock Analysis Report
