Legend (LEGN) Up 4% on sNDA Submission for Carvykti Expanded Use
Legend Biotech Corporation LEGN announced that it submitted a supplemental biologics license application (sNDA) to the FDA for Carvykti (ciltacabtagene autoleucel; cilta-cel). The sNDA seeks to expand Carvykti’s label to include the treatment of adult patients with relapsed and lenalidomide-refractory multiple myeloma who have received at least one prior line of therapy. The stock of the company rose 4.2% on Tuesday in response to the positive news.
Carvykti is a B-cell maturation antigen-directed genetically modified autologous T cell immunotherapy. It is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma, after four or more prior lines of therapy, including a proteasome inhibitor, an immunomodulatory agent and anti-CD38 monoclonal antibody.
The drug has been developed by the company in collaboration with J&J JNJ. Legend Biotech entered into an exclusive worldwide license and collaboration agreement with J&J to develop and commercialize cilta-cel in December 2017.
Cilta-cel was first approved by the FDA in February 2022, under the brand name, Carvykti, for the treatment of adults with relapsed or refractory multiple myeloma. Carvykti was granted conditional marketing authorization by the European Commission, for the same indication, in May 2022.
So far this year, shares of LEGN have gained 36.4% against the industry’s 7.3% fall.
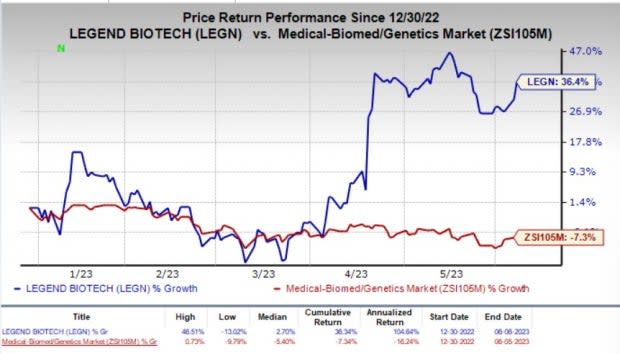
Image Source: Zacks Investment Research
The sNDA submission for Carvykti is based on results from Legend and J&J’s phase III CARTITUDE-4 study of the drug. The companies reported positive top-line results from the study in January 2023. The phase III CARTITUDE-4 study evaluated the efficacy and safety of chimeric antigen receptor (CAR)-T therapy against available standard-of-care therapies in adult patients with relapsed and lenalidomide-refractory multiple myeloma who received one to three prior lines of therapy.
The study met its primary endpoint of progression-free survival with statistical significance. Secondary endpoints include safety, overall survival, minimal residual disease negative rate and overall response rate. Patients will continue to be followed for primary and secondary endpoints as part of the CARTITUDE-4 study.
Earlier this week, Legend Biotech announced that results from the phase III CARTITUDE-4 study suggest treatment with Carvykti cut the risk of death or disease progression by 74% compared with the current standard of care in patients who had tried one to three lines of therapyand are refractory to Revlimid.
Multiple myeloma is a fatal blood cancer that originates in the bone marrow and characterized by an excessive proliferation of plasma cells. More than 35,000 people in the United States are estimated to be diagnosed with this disease in 2023.
However, there are other players in the market like Bristol Myers BMY and 2seventy bio TSVT, with a drug having the same mechanism of action. Legend Biotech and J&J face significant competition from Bristol Myers and partner, 2seventy bio’s Abecma.
In March 2021, Abecma received FDA approval as the first B-cell maturation antigen-directed CAR-T cell immunotherapy for the treatment of adult patients with relapsed or refractory multiple myeloma after four or more prior lines of therapy. BMY and TSVT are jointly involved in developing and commercializing Abecma in the United States as part of the collaboration agreement. Abecma received approval from the European Medicines Agency in August 2021, for use in European Union for the same indication.
Legend Biotech Corporation Sponsored ADR Price and Consensus
Legend Biotech Corporation Sponsored ADR price-consensus-chart | Legend Biotech Corporation Sponsored ADR Quote
Zacks Rank
Legend Biotech currently has a Zacks Rank #3 (Hold).
You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Bristol Myers Squibb Company (BMY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Legend Biotech Corporation Sponsored ADR (LEGN) : Free Stock Analysis Report
2seventy bio, Inc. (TSVT) : Free Stock Analysis Report
