Lilly's (LLY) Eczema Candidate Lebrikizumab Faces FDA Rejection
Eli Lilly and Company LLY announced that the FDA has issued a complete response letter (CRL) to its biologic license application (BLA) seeking the approval of pipeline candidate, lebrikizumab, for moderate-to-severe atopic dermatitis, also called eczema.
The CRL was based on inspection findings at a third-party, contract manufacturer, which included a monoclonal antibody drug substance for lebrikizumab. The CRL did not mention any issues with the clinical data that supported the BLA or the safety or label of the candidate.
Lilly plans to work closely with the FDA and the third-party manufacturer to resolve the issue.
The BLA includes data from Advocate 1, ADvocate 2 and ADhere studies on lebrikizumab. A regulatory application seeking the approval of lebrikizumab is also under review in Europe, with a decision expected later this year.
The stock has risen 47.1% year to date compared with an increase of 4.2% of the industry.

Image Source: Zacks Investment Research
In addition to lebrikizumab, Lilly has a solid pipeline of drug candidates, of which some are already approved, while regulatory decisions on some other candidates are expected later this year.
Among other key pipeline candidates is Omvoh/mirikizumab, which is under review in the United States for ulcerative colitis while already launched in Japan and the EU, with planned additional launches in the EU later this year. Lilly expects the FDA decision on Omvoh/mirikizumab in the United States later in 2023.
Jaypirca/pirtobrutinib, a BTK inhibitor, was approved for mantle cell lymphoma (MCL) in the United States in January 2023, while it is under review in Europe for the MCL indication. The FDA’s decision on Jaypirca for another indication, chronic lymphocytic leukemia, is expected later this year.
An important pipeline candidate is donanemab, which is under review in the United States and Europe for early Alzheimer’s disease. The FDA decision on donanemab is expected later this year. Lilly expects approval of donanemab based on promising data from the TRAILBLAZER-ALZ 2 study, which formed the basis of Lilly’s BLA application for traditional regulatory approval. Data from the study showed that donanemab treatment slowed the clinical decline in Alzheimer's by 35%.
An important new drug in Lilly’s portfolio is Mounjaro, a dual GIP and GLP-1 receptor agonist, which was approved for type II diabetes in 2022 and is already generating impressive sales. Mounjaro sales totaled $1.55 billion in the first half of 2023.
Moreover, Mounjaro showed a superior weight-loss reduction in clinical studies for the obesity indication. Regulatory applications have already been filed for Mounjaro for the obesity indication in the United States and the EU. In the United States, the FDA has assigned priority review to the regulatory filing, with a decision expected by year-end.
Zacks Rank & Stocks to Consider
Lilly currently has a Zacks Rank #3 (Hold).
Eli Lilly and Company Price and Consensus
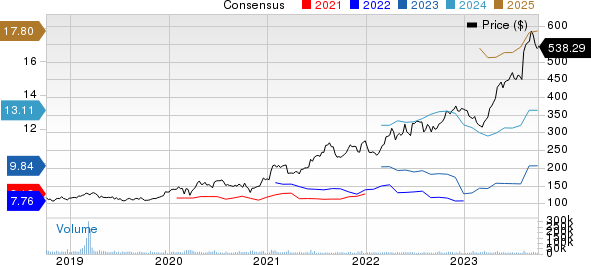
Eli Lilly and Company price-consensus-chart | Eli Lilly and Company Quote
Some better-ranked drug/biotech companies worth considering are Alpine Immune Sciences ALPN, Aurinia Pharmaceuticals AUPH and Corcept Therapeutics CORT, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 60 days, the consensus estimate for Alpine Immune Sciences’ 2023 loss has narrowed from $1.43 per share to $1.18 per share, while that for 2024 has narrowed from $1.73 per share to $1.47 per share. Year to date, shares of Alpine Immune Sciences have rallied 54.7%.
ALPN’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average negative surprise of 79.65%.
In the past 60 days, the consensus mark for Aurinia Pharmaceuticals’ 2023 loss has narrowed from 71 cents per share to 58 cents per share, while that for 2024 has narrowed from 43 cents to 27 cents. Year to date, shares of Aurinia Pharmaceuticals have surged 72.2%.
The earnings of Aurinia Pharmaceuticals beat estimates in all the last four quarters, delivering an earnings surprise of 45.61% on average.
In the past 60 days, the Zacks Consensus Estimate for Corcept’s earnings has increased from 62 cents per share to 78 cents for 2023. The bottom-line estimate has also improved from 61 cents to 83 cents for 2024 during the same period. Shares of the company have rallied 29.4% year to date.
CORT’s earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, delivering an average surprise of 6.99%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Corcept Therapeutics Incorporated (CORT) : Free Stock Analysis Report
Aurinia Pharmaceuticals Inc (AUPH) : Free Stock Analysis Report
Alpine Immune Sciences, Inc. (ALPN) : Free Stock Analysis Report
