Liquidia (LQDA) Up on Deal With Pharmosa to Co-Develop PH Drug
Liquidia Corporation LQDA has entered into an exclusive licensing agreement with Pharmosa Biopharm for the development and commercialization of L606 in North America. L606 is an inhaled, twice-daily sustained-release formulation of Treprostinil, which is currently being evaluated in a late-stage study for the treatment of pulmonary arterial hypertension (PAH) and pulmonary hypertension associated with interstitial lung disease (PH-ILD).
Per the terms of the agreement, Liquidia will gain sole rights to develop and conduct regulatory and commercial activities of L606 in North America. On the other hand, Pharmosa will manufacture and supply L606 to Liquidia for clinical and commercial purposes. LQDA is liable to make an upfront payment of $10 million to Pharmosa for the transfer of the exclusive rights to L606. Pharmosa is also eligible to receive up to $215 million on achieving certain development and sales-based milestones, tied to PAH and PH-ILD indications, and two tiers of low, double-digit royalties on net sales of L606.
Shares of the company climbed 11% on Wednesday in response to the encouraging collaboration deal. Year to date, shares of Liquidia have gained 55.4% against the industry’s 9.7% fall.
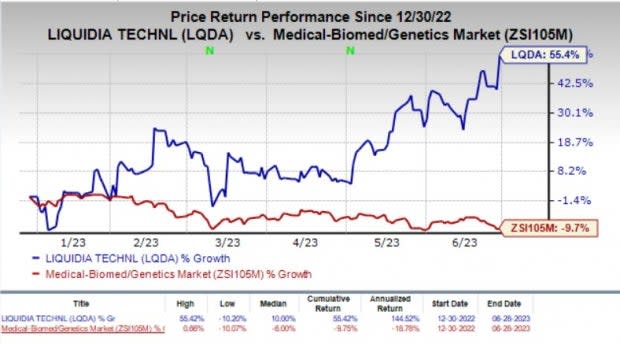
Image Source: Zacks Investment Research
Furthermore, Pharmosa is also entitled to receive a $10 million milestone payment for each additional indication and additional product approved. Liquidia has retained the right of first negotiation in partnering discussions for the development and commercialization of L606 in Europe and other territories should Pharmosa seek a partner, subject to the satisfaction of certain conditions stated in the license agreement.
Liquidia intends to file the first regulatory approval for L606 in the United States. The new drug application for L606 is expected to be supported by three sets of data. First will be the results obtained from the completed phase I study of L606, which showed tolerability and comparable pharmacokinetics to nebulized Tyvaso (treprostinil) inhalation solution. It will also contain clinical data from the ongoing phase III study on L606 in the treatment of PAH and PH-ILD in the United States. Finally, the company will also include data from a pivotal study evaluating L606 in the treatment of PH-LD patients, which is expected to begin in the first half of 2024.
LQDA also reported that the encouraging collaboration agreement has triggered another $10 million from Liquidia’s financing agreement with HealthCare Royalty (HCRx). This influx of cash will benefit the company to make the upfront payment to Pharmosa. Total proceeds funded to Liquidia by HCRx have now amounted to $42.5 million of the up to $100 million contemplated by the financing agreement with the two companies.
In the same press release, Liquidia also stated that it is gearing up for the potential launch of its lead product candidate, Yutrepia (treprostinil) inhalation powder. Yutrepia is an investigational, inhaled dry powder formulation of Treprostinil, which received tentative approval from the FDA in November 2021 for treating PAH. The company also reported that the FDA has confirmed that Yutrepia may add the indication to treat PH-ILD without conducting additional clinical studies.
With continued investments in the anticipated commercial launch of Yutrepia and the collaboration agreement with Pharmosa, Liquidia expects to address the serious unmet medical need in treating PH and provide better treatment options to the patients.
Liquidia Technologies, Inc. Price and Consensus

Liquidia Technologies, Inc. price-consensus-chart | Liquidia Technologies, Inc. Quote
Zacks Rank and Stocks to Consider
Liquidia currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Adaptimmune Therapeutics ADAP, Akero Therapeutics AKRO and ADMA Biologics, Inc. ADMA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has narrowed from 63 cents to 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from 59 cents to 56 cents. Year to date, shares of ADAP have fallen by 37.6%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
In the past 90 days, the Zacks Consensus Estimate for Akero Therapeutics’ 2023 loss per share has narrowed from $2.96 to $2.80. During the same period, the estimate for Akero Therapeutics’ 2024 loss per share has narrowed from $3.40 to $3.27. Year to date, shares of AKRO have lost 17.1%.
AKRO beat estimates in three of the trailing four quarters, missing the mark on one occasion, delivering an average earnings surprise of 7.96%.
In the past 90 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 14 cents to 9 cents. The consensus estimate for 2024 earnings is currently pegged at 7 cents per share. Year to date, shares of ADMA have lost 1%.
ADMA beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 19.13%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report
Liquidia Technologies, Inc. (LQDA) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
