Medtronic (MDT) Starts Pediatric Trial for Scoliosis Treatment
Medtronic plc MDT recently announced the enrollment of its first patient and completion of the first surgical procedure under its BRAIVE IDE study. The study will assess the safety and efficacy of the Braive growth modulation system for treatment of progressive Adolescent Idiopathic Scoliosis (AIS).
This device is Medtronic's latest advancement in the pediatric spine category. The initiation of the BRAIVE IDE study reaffirms the company's commitment to continued innovation for pediatric patients.
The recent development is likely to fortify Medtronic’s Cranial & Spinal Technologies business, which is part of the Neuroscience portfolio.
More on the Device and Study
The Braive system is intended to correct scoliosis while enabling the spine to continue to grow, which is vital for adolescents experiencing their most important period of growth.
The Braive growth modulation system utilizes a braid secured to the spine with screws to slow growth on the curved side of the spine, while enabling growth to continue on the other side.
The BRAIVE IDE study will assess the safety and effectiveness in correcting the spine's curve in patients with juvenile or adolescent idiopathic scoliosis. The forthcoming, multi-center study will enroll patients in the United States, Canada, and the United Kingdom. The first patient was employed by The Newcastle Upon Tyne Hospitals NHS Foundation Trust, U.K.
Significance of the Study
It is worth mentioning that globally, nearly 4% of children have scoliosis, making it one of the most frequent pediatric orthopedic deformities. According to the National Scoliosis Foundation, an anticipated 30,000 children per year receive a brace to treat their condition, while 38,000 patients are treated with spinal fusion. Despite the success in correcting the spine's curves successfully, spinal fusion causes vertebrae to combine into a single bone, which impedes growth in that area of the spine.
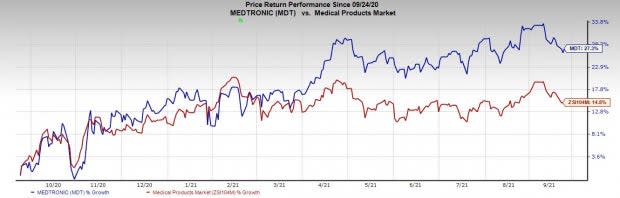
Image Source: Zacks Investment Research
Per Medtronic’s management, introducing the BRAIVE IDE study is the newest step in bringing life-changing technologies to pediatric patients. As image guidance and navigation compatibilities broaden into additional spinal implant systems designated for pediatric populations, they are combined with a fast cadence of transformative implant innovation. This allows the company to deliver the most all-inclusive and integrated ecosystem of procedural solutions to pediatric spine surgeons that can lead to significant advancements in clinical outcomes for young patients.
Industry Prospects
Per a report by Market Watch, the global scoliosis treatment market size is projected to reach $26.49 billion by 2027, from $22.05 billion in 2020, at a CAGR of 2.2%.
The greater adoption of implants in orthopedic, an increasing incidence of congenital and idiopathic scoliosis mainly in adolescents are the factors driving the market.
Notable Developments
In August 2021, Medtronic entered into a definitive agreement with Intersect ENT XENT, wherein the former has agreed to acquire Intersect ENT for $1.10 billion. The move is intended to expand Medtronic's product portfolio for ear, nose, and throat procedures while specifically targeting the chronic rhinosinusitis market (CRS). Intersect ENT’s complementary product lines and customer base will advance Medtronic's efforts to offer best solutions for patients suffering from CRS.
In June 2021, Medtronic announced the receipt of the FDA approval for Vanta -- a high performance recharge-free implantable neurostimulator (INS) with a device life that can be optimized up to 11 years. The extended battery life, broad MRI compatibility and personalized relief through AdaptiveStim technology enable a more hassle-free experience and greater freedom for patients to manage chronic pain.
Price Performance
Shares of the company have gained 27.3% in a year’s time compared with the industry’s rise of 14.8%.
Zacks Rank and Key Picks
Currently, Medtronic carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks from the broader medical space are Envista Holdings Corporation NVST and BellRing Brands, Inc. BRBR, each carrying a Zacks Rank #2 (Buy). You can see the complete list of Zacks #1 Rank (Strong Buy) stocks here.
Envista Holdings has an estimated long-term earnings growth rate of 27%.
BellRing Brands has an estimated long-term earnings growth rate of 29%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Medtronic PLC (MDT) : Free Stock Analysis Report
Intersect ENT, Inc. (XENT) : Free Stock Analysis Report
Envista Holdings Corporation (NVST) : Free Stock Analysis Report
BellRing Brands, Inc. (BRBR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
