Merck's (MRK) Gastric Cancer Study Fails to Meet a Primary Goal
Merck MRK announced that the phase III KEYNOTE-585 study, evaluating Keytruda in an advanced gastric cancer indication, failed to achieve statistical significance in one of the primary endpoints of event-free survival (“EFS”).
The KEYNOTE-585 study evaluated the combination of Keytruda and chemotherapy (“Keytruda regimen”) against placebo in patients with locally advanced resectable gastric and gastroesophageal junction adenocarcinoma. The primary endpoints of the study were EFS, overall survival (“OS”) and pathological complete response (“pCR”).
Data from the study showed that participants treated with the Keytruda regimen achieved statistically significant improvement in pCR endpoint, which measures the absence of all signs of cancer in tissue samples.
Since the study was unable to achieve the EFS endpoint, the OS endpoint was not formally tested.
Shares of Merck have nosedived 0.7% year to date against the industry’s 1.9% growth.
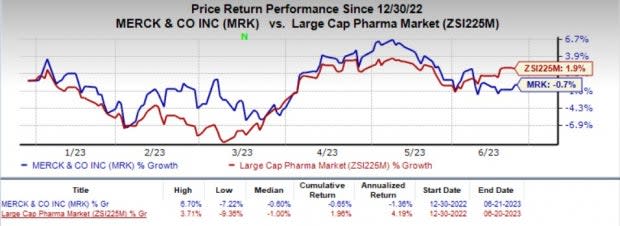
Image Source: Zacks Investment Research
The detailed results of the KEYNOTE-585 study will be presented at a future medical meeting.
Keytruda has received global approval for treating various types of cancer.
The drug’s sales are gaining from continued strong momentum in metastatic indications, including some types of non-small cell lung cancer, renal cell carcinoma, head and neck squamous cell carcinoma cancers, and a rapid uptake across recent earlier-stage launches. It is also being studied for over 30 different types of cancer, as both monotherapy and combination studies.
Keytruda is continuously growing and expanding into new indications and markets globally.
Earlier this month, Merck announced that the FDA accepted its supplemental biologics license application, seeking approval for Keytruda combined with the standard-of-care chemotherapy to treat patients diagnosed with locally advanced unresectable or metastatic biliary tract cancer.
A final decision from the regulatory body is expected by Feb 7, 2024.
Merck & Co., Inc. Price and Consensus
Merck & Co., Inc. price-consensus-chart | Merck & Co., Inc. Quote
Zacks Rank and Stocks to Consider
Merck currently has a Zacks Rank #3 (Hold).
Some better-ranked stocks in the biotech sector are Akero Therapeutics AKRO, ADMA Biologics, Inc. ADMA and Omega Therapeutics OMGA, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
The Zacks Consensus Estimate for Akero Therapeutics has narrowed from a loss of $2.96 to a loss of $2.80 for 2023 in the past 90 days. Shares of Akero Therapeutics have declined 0.4% year to date.
AKRO’s earnings beat estimates in three of the trailing four quarters and missed the mark in one, delivering an average surprise of 7.96%.
The consensus estimate for ADMA Biologics has narrowed from a loss of 19 cents to a loss of 9 cents for 2023 in the past 90 days. Shares of ADMA Biologics have risen 1.3% year to date.
ADMA’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 19.13%.
The consensus estimate for Omega Therapeutics has narrowed from a loss of $2.51 to a loss of $2.05 for 2023 in the past 90 days. Shares of the company have rallied 31.1% year to date.
OMGA’s earnings beat estimates in two of the trailing four quarters, met the mark in one and missed in another, delivering an average surprise of 8.24%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report
Omega Therapeutics, Inc. (OMGA) : Free Stock Analysis Report
