Mersana (MRSN) Down on Partial Ovarian Cancer Study Hold
Mersana MRSN plunged 59% as it announced that the FDA has placed a partial clinical hold on the enrollment of new patients in two of its ongoing clinical studies — UP-NEXT and UPGRADE-A. These studies were evaluating the efficacy of Mersana's lead product candidate, upifitamab rilsodotin (UpRi), in platinum-sensitive ovarian cancer.
The FDA's decision to pause new patient enrollment in phase III UP-NEXT study and phase I UPGRADE-A study followed the company’s submission of an aggregate safety report that evaluated bleeding events in approximately 560 patients who received UpRi.
The report indicated that there was a higher number of serious bleeding events compared with the expected background rate of bleeding in platinum-resistant ovarian cancer. Although most instances of bleeding were categorized as low-grade, the report identified five fatal Grade 5 bleeding events among patients treated so far. The causes of these bleeding events are being investigated.
As part of the partial clinical hold, the FDA is expected to request a comprehensive assessment of the safety data associated with UpRi, including the bleeding events.
Mersana’s phase II UPLIFT study of UpRi for platinum-resistant ovarian cancer remains untouched. The study finished enrolling patients in October 2022. Patients who were already enrolled in the study can still receive UpRi. The company plans to secure the UPLIFT study information and share its results by early August 2023.
The company aims to work with the FDA and address any concerns to lift the partial clinical hold and resume patient enrollment in UP-NEXT and UPGRADE-A.
Shares of Mersana have plunged 33.6% year to date compared with the industry’s 7.1% decline.
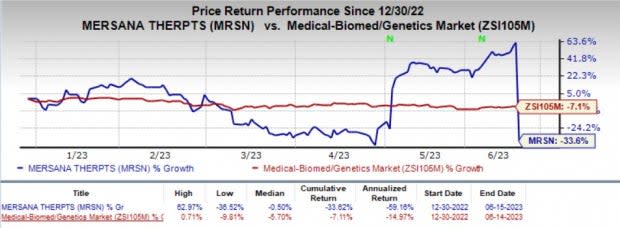
Image Source: Zacks Investment Research
This partial clinical hold represents a temporary setback for Mersana and its efforts to advance the development of UpRi.
In February 2022, Mersana partnered with Janssen Biotech, a subsidiary of Johnson and Johnson JNJ, in a research collaboration and license agreement. The agreement aims to develop and market new ADCs for three types of cancer. It will do so using MRSN’s expertise in ADCs and Janssen's antibodies.
As part of the agreement, Janssen made an initial payment of $40.0 million to Mersana. Janssen has the option to choose up to three targets for the treatments. It can also change each target once before a specific deadline.
Mersana Therapeutics, Inc. Price and Consensus
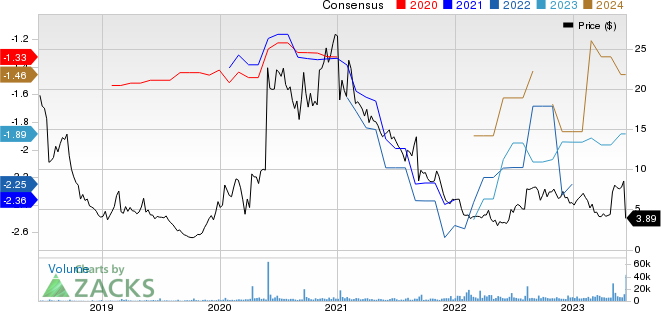
Mersana Therapeutics, Inc. price-consensus-chart | Mersana Therapeutics, Inc. Quote
Zacks Rank and Stocks to Consider
Mersana currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the biotech sector are ADMA Biologics, Inc. ADMA and Omega Therapeutics OMGA, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Loss per share estimate for ADMA Biologics has narrowed from 19 cents to 9 cents for 2023 in the past 90 days. Shares of ADMA Biologics have risen 2.9% year to date.
ADMA’s earnings beat estimates in each of the trailing four quarters, delivering an average surprise of 19.13%.
Loss per share estimate for Omega Therapeutics has narrowed from $2.51 to $2.05 for 2023 in the past 90 days. Shares of the company have rallied 39.2% year to date.
OMGA’s earnings beat estimates in two of the trailing four quarters, met the mark in one and missed in another, delivering an average surprise of 8.24%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Mersana Therapeutics, Inc. (MRSN) : Free Stock Analysis Report
Omega Therapeutics, Inc. (OMGA) : Free Stock Analysis Report
