Milestone Pharma (MIST) Down on Regulatory Update for Etripamil
Milestone Pharmaceuticals MIST announced that the FDA issued a Refusal to File (RTF) letter for the new drug application (NDA) seeking approval for self-administered etripamil nasal spray for the treatment of paroxysmal supraventricular tachycardia (PSVT).
Per the FDA, the NDA for etripamil to treat PSVT, submitted in October 2023, was not sufficiently complete to permit substantive review. The regulatory body has requested clarification about the time of data recorded for adverse events related to treatment with etripamil in phase III studies supporting the NDA.
However, Milestone Pharma stated that the FDA has not shown any concern about the nature or severity of the adverse events. The company is currently preparing to meet with the FDA to seek clarification for the RTF issued to the NDA seeking approval for etripamil to treat PSVT and figure out a way forward.
Milestone Pharma’s stock plunged 30.9% in the last trading session as investors were disappointed by the unfavorable regulatory update on the NDA for etripamil to treat PSVT. Year to date, MIST shares have plummeted 49.7% compared with the industry’s 16.1% fall.
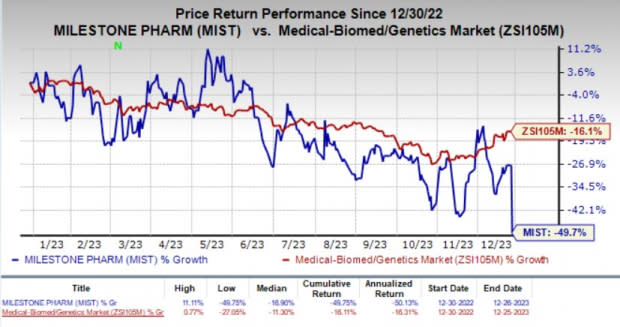
Image Source: Zacks Investment Research
Etripamil, a novel calcium channel blocker nasal spray, is the company’s lead and only investigational candidate. The FDA has approved a conditional brand name for etripamil, Cardamyst.
The NDA submission was based on positive results from the completed phase III RAPID study of etripamil, evaluating the safety and efficacy of the candidate for treating PSVT compared with placebo. The late-stage study met its primary endpoint observing 64% of patients who self-administered etripamil converted from supraventricular tachycardia to sinus rhythm within 30 minutes compared with 31% on placebo.
At the one-hour mark, the benefit was observed in 73% of the patients who received etripamil. Additionally, patients who took etripamil achieved significant reductions in time to conversion, which were evident early and durable. The median time to conversion for patients treated with etripamil was 17 minutes compared with 54 minutes for patients treated with placebo.
The company further stated that the data from the RAPID study demonstrated statistically significant improvement in multiple defined symptoms of PSVT in patients receiving etripamil compared with placebo, determined using a patient-reported outcome questionnaire.
PSVT is a type of arrhythmia or abnormal heart rhythm and is characterized by episodes of rapid heartbeats often exceeding 150 to 200 beats per minute. Per Milestone Pharma, approximately two million people suffer from PSVT in the United States. This represents a significant unmet medical need.
Subject to successful development, etripamil nasal spray has the potential to become the first-of-its-kind portable and fast-acting solution that would allow patients to self-administer without being admitted to a hospital.
MIST is also simultaneously developing the etripamil nasal spray for a second indication. A phase II study is currently evaluating the candidate for the treatment of patients with atrial fibrillation with rapid ventricular rate.
At present, the company does not have a pipeline beyond the etripamil nasal spray development program.
Milestone Pharmaceuticals Inc. Price and Consensus
Milestone Pharmaceuticals Inc. price-consensus-chart | Milestone Pharmaceuticals Inc. Quote
Zacks Rank and Stocks to Consider
Milestone Pharma currently has a Zacks Rank #3 (Hold).
Some better-ranked drug/biotech stocks are Puma Biotechnology, Inc. PBYI, ADMA Biologics ADMA and Agenus AGEN. PBYI sports a Zacks Rank #1 (Strong Buy), and ADMA and AGEN carry a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 30 days, the Zacks Consensus Estimate for Puma Biotech’s 2023 earnings per share (EPS) has decreased from 73 cents to 72 cents. During the same time frame, the consensus estimate for Puma Biotech’s 2024 EPS has increased from 62 cents to 64 cents. In the year so far, shares of PBYI have gained 5.4%.
PBYI’s earnings beat estimates in three of the last four quarters while missing on one occasion, delivering a four-quarter average earnings surprise of 76.55%.
In the past 30 days, the Zacks Consensus Estimate for ADMA Biologics’ 2023 loss per share has narrowed from 3 cents to 2 cents. The consensus estimate for ADMA Biologics’ 2024 EPS is pegged at 18 cents. In the year so far, shares of ADMA have gained 12.4%.
ADMA beat estimates in three of the trailing four quarters and matched in one, delivering an average earnings surprise of 63.57%.
In the past 30 days, the Zacks Consensus Estimate for Agenus’ 2023 loss per share has been constant at 63 cents. During the same time frame, the consensus estimate for Agenus’ 2024 loss per share has been constant at 45 cents. In the year so far, AGEAN shares have lost 65.8%.
AGEN beat estimates in one of the trailing four quarters, matching in one and missing the mark on the other two occasions, delivering an average earnings surprise of 0.49%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Agenus Inc. (AGEN) : Free Stock Analysis Report
Puma Biotechnology, Inc. (PBYI) : Free Stock Analysis Report
ADMA Biologics Inc (ADMA) : Free Stock Analysis Report
Milestone Pharmaceuticals Inc. (MIST) : Free Stock Analysis Report
