Moderna (MRNA) Gets CHMP Nod for Omicron BA.4, BA.5 Booster Jab
Moderna MRNA announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (“CHMP”) recommended approving the use of a 50-µg booster dose of its mRNA-based bivalent BA.4/BA.5 Omicron-targeting COVID-19 vaccine, mRNA-1273.222. The dose will be administered to individuals 12 years of age and older.
This bivalent vaccine combines 25-µg Spikevax (mRNA-1273), Moderna’s currently marketed COVID vaccine, and 25-µg of another vaccine candidate targeting the Omicron BA.4 and BA.5 subvariants.
The CHMP recommendation is supported by data based on preclinical studies conducted on mRNA-1273.222 and data from the phase II/III study, which evaluated mRNA-1273.214, another bivalent candidate developed by Moderna to target the Omicron BA.1 subvariant. mRNA-1273.214 was authorized by European Commission (“EC”) last month for individuals aged 12 years and above.
An ongoing phase II/III study is evaluating mRNA-1273.222. Initial data from the same is expected by this year’s end.
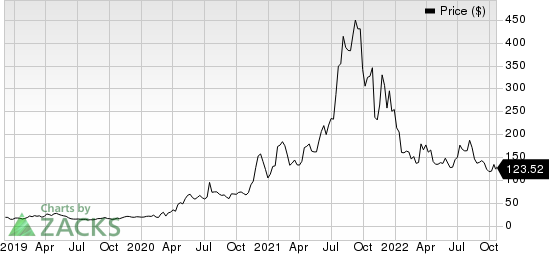
Shares of Moderna have declined 51.4% so far this year compared with the industry’s 25.0% fall.

Image Source: Zacks Investment Research
If mRNA-1273.222 were authorized, it would face stiff competition from the mRNA-based bivalent vaccines developed by Pfizer PFE and its partner BioNTech BNTX. Pfizer/BioNTech received authorization for two bivalent vaccines targeting the Omicron variant. While Pfizer/BioNTech initially received authorization for its BA.1 Omicron-targeting bivalent vaccine on the same day as mRNA-1273.214, the EC authorized their Omicron BA.4/BA.5-adapted bivalent vaccine in adults and adolescents on Sep 12, 2022.
Currently, the bivalent BA.4/BA.5 Omicron-targeting COVID-19 vaccines developed by Moderna and Pfizer/BioNTech are authorized for use in the United States. While Pfizer/BioNTech’s vaccine is authorized for use in individuals aged five years and older, Moderna’s bivalent vaccines can be administered to individuals aged six years and above.
In a separate press release, Moderna also announced that the CHMP recommended 25-µg two-dose series of Spikevax for use in individuals aged six months to five years of age. The CHMP has also recommended expanding the use of Pfizer/BioNTech’s Comirnaty for use in individuals aged between six months and four years of age. Currently, a primary regimen of Spikevax is authorized for use in people aged six years and older, while Comirnaty is authorized for use in individuals aged five years and older.
Moderna, Inc. Price
Moderna, Inc. price | Moderna, Inc. Quote
Zacks Rank & Key Pick
Moderna currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the overall healthcare sector is Morphic MORF, which sports a Zacks Rank #1 (Strong Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
In the past 60 days, estimates for Morphic’s 2023 loss per share have narrowed from $3.77 to $3.61 during the same period. Shares of Morphic have lost 48.2% in the year-to-date period.
Earnings of Morphic beat estimates in three of the last four quarters and missed the mark just once, witnessing a surprise of 48.29%, on average. In the last reported quarter, MORF delivered an earnings surprise of 183.95%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Morphic Holding, Inc. (MORF) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
