Novavax's (NVAX) Updated COVID Jab Gets WHO Nod for Emergency Use
Novavax NVAX announced that the World Health Organization (“WHO”) has granted emergency use listing (“EUL”) to NVX-CoV2601, its updated protein-based COVID vaccine, for use in individuals aged 12 years and older.
The COVID-19 vaccines that are granted EUL meet WHO standards for quality, safety and efficacy. Furthermore, this listing helps the WHO member states in assessing vaccines with the aim of expediting availability and regulatory approvals for the vaccine.
The EUL is based on data from non-clinical studies wherein Novavax’s updated vaccine generated functional immune responses against multiple circulating Omicron-related sub-lineages, including XBB.1.5, BA.2.86 (also called ‘Pirola’), EG.5.1 (also called ‘Eris’), FL.1.5.1 (also called ‘Fornax’) and XBB.1.16.6.
Shares of Novavax rose 5% on Tuesday following this news announcement. This will likely benefit Novavax, whose updated COVID-19 vaccine will be available as an option to all 194 WHO member states. With Novavax currently struggling with cash flow needs, a surge for COVID-19 vaccines will help the company generate additional cash resources to fund its business operations. In an SEC filing in November, management stated that they have sufficient cash to fund business for the next 12 months.
So far, Novavax’s stock has lost 44.3% year to date compared with the industry’s 23.5% decline.
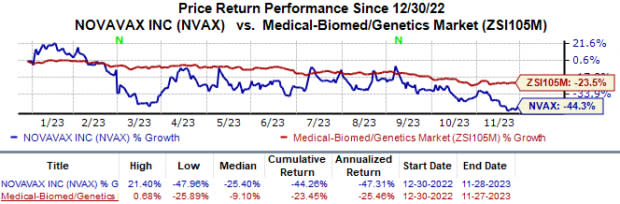
Image Source: Zacks Investment Research
Last month, the FDA granted emergency use authorization (“EUA”) to NVX-CoV2601 in individuals aged 12 years and older. Following the EUA, Novavax’s updated vaccine is the only non-mRNA vaccine option available to people in the United States.
Despite being one of the few companies with an authorized vaccine for COVID-19 in the United States, Novavax significantly missed out on the COVID-19 vaccine windfall, which benefitted the mRNA vaccine makers Pfizer PFE/BioNTech BNTX and Moderna MRNA. At the time when the pandemic was at its peak, Novavax faced manufacturing issues, which in turn delayed its regulatory filing timeline.
In September, the FDA approved Moderna’s and Pfizer/BioNTech’s updated mRNA vaccines for use in individuals aged 12 years and older. The Moderna and Pfizer/BioNTech vaccines also hold an edge over Novavax’s vaccine since they have also been granted EUA for individuals aged six months through 11 years.
Unlike previous times when the government handled the distribution and rollout of COVID-19 vaccines, this year onward, the vaccines have been launched for the first time via a commercial rollout through retail pharmacies and physician offices. The updated COVID vaccines are supposed to be accessible through private insurance, Medicare and Medicaid plans.
For those who are uninsured or underinsured, the CDC will temporarily provide free shots through its Bridge Access Program.
Novavax, Inc. Price
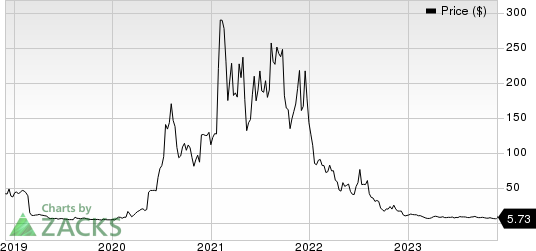
Novavax, Inc. price | Novavax, Inc. Quote
Zacks Rank
Novavax currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report
Novavax, Inc. (NVAX) : Free Stock Analysis Report
BioNTech SE Sponsored ADR (BNTX) : Free Stock Analysis Report
